Galvanic Cells
Quick Notes
- A Galvanic Cell converts chemical energy from a redox reaction into electrical energy.
- Zinc and Copper electrodes dipped in their respective salt solutions form a typical galvanic cell.
- Salt Bridge maintains charge neutrality by allowing ions to flow freely into each half-cell and balance charge.
- Anode: Site of oxidation (Zn → Zn2+ + 2e−)
- Cathode: Site of reduction (Cu2+ + 2e− → Cu)
- Electrons flow from anode to cathode through the external circuit.
- Salt bridge completes the circuit internally via ion migration.
- Cell Notation: Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
- Electrode potential is measured by connecting an electrode to the Standard Hydrogen Electrode (SHE).
Full Notes
What exactly is a Voltaic Cell?
A voltaic (or galvanic) cell is a type of electrochemical cell in which a spontaneous redox reaction generates an electric current.
A simple voltaic cell can be constructed using two half-cells connected by a salt bridge and an external wire.
Half-Cells
Each half-cell consists of:
- A metal electrode (solid metal)
- An electrolyte (a solution containing ions of that metal)
A redox equilibrium is established between the metal atoms and their ions:
Mn+(aq) + ne− ⇌ M(s)
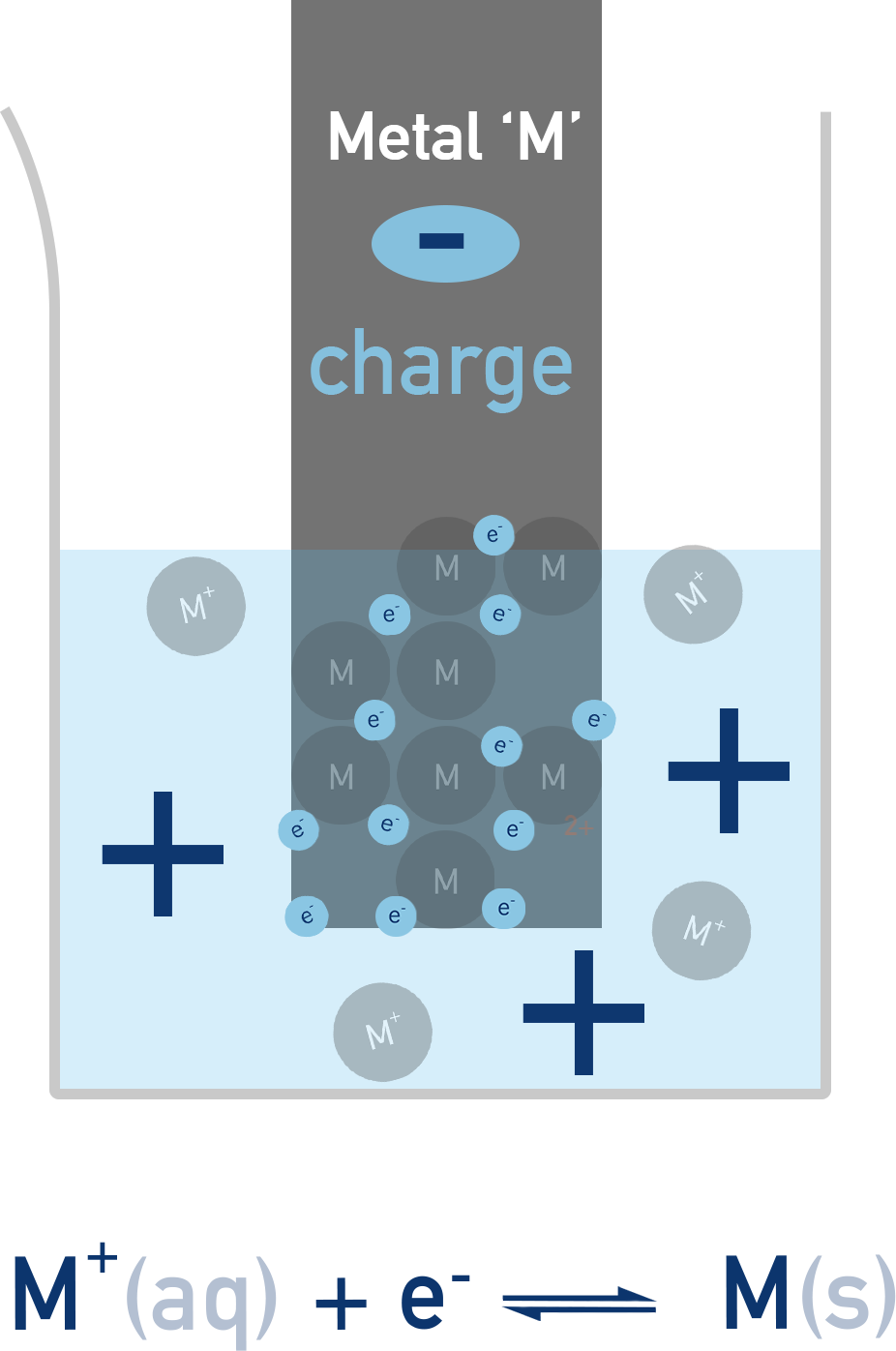
This sets up a potential difference between the metal and the solution. The position of equilibrium – and hence the total charge or ‘potential’ of the electrode – depends on how readily the metal loses or gains electrons.
The electrode potential of a half-cell cannot be measured directly, but we can compare it to a standard reference (like the standard hydrogen electrode) to determine its relative value.
Components of a Voltaic Cell
- Anode: site of oxidation
- Metal loses electrons and enters solution as ions
- Electrons flow away from the anode
- Cathode: site of reduction
- Metal ions in solution gain electrons and are deposited as metal
- Electrons flow into the cathode
- Electron flow: Anode → external circuit → Cathode
Salt Bridge Function
The salt bridge allows ion exchange between half-cells.
- Prevents charge buildup by allowing:
- Cations to migrate toward the cathode
- Anions to migrate toward the anode
- Common salt bridge solution: KNO3 or Na2SO4
How a simple cell works: Zn–Cu Voltaic Cell
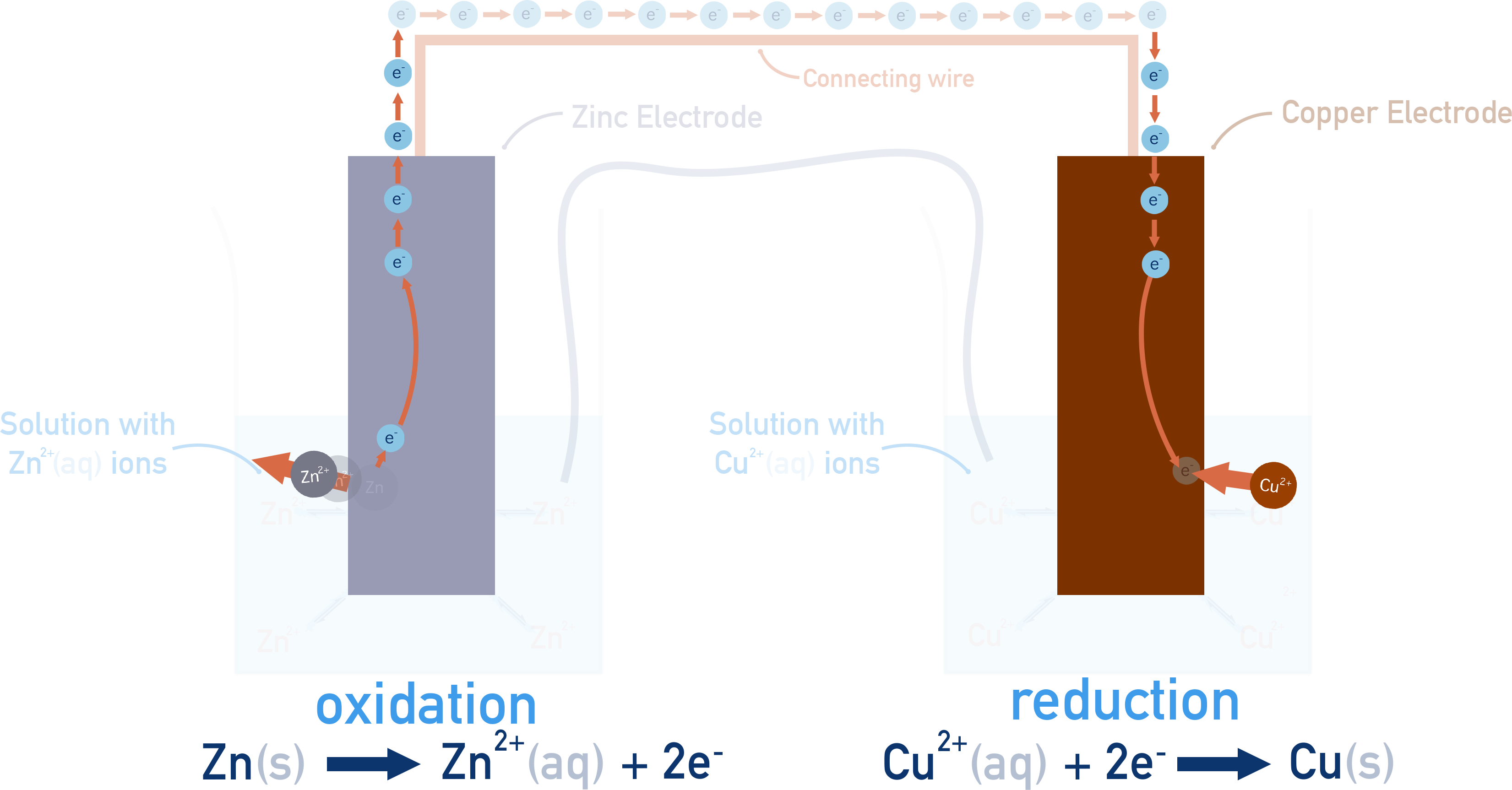
At the anode, zinc metal is oxidised:
Zn(s) → Zn2+(aq) + 2e−
The released electrons travel through the external circuit to the cathode, where Cu2+ ions are reduced:
Cu2+(aq) + 2e− → Cu(s)
As the reaction proceeds, Zn metal is gradually consumed, and solid copper builds up on the cathode. Electron flow from anode to cathode continues as long as zinc is available to oxidise and Cu2+ ions remain to be reduced.
The salt bridge maintains charge balance by allowing ions to move between the two half-cells. This prevents charge buildup, which would otherwise stop the redox reactions from continuing.
Cell Notation
In electrochemistry, cells are written using a shorthand:
- A single vertical line (|) separates different phases (solid, liquid, aqueous).
- A double line (||) represents the salt bridge.
- The anode (oxidation) is written on the left, and the cathode (reduction) on the right.
For Example:
For write the conventional cell notation for an electrochemical cell made from two half cells made up of the following:
Zn2+(aq) + 2e− ⇌ Zn(s)
Cu2+(aq) + 2e− ⇌ Cu(s)
We’ve already established (see above) Cu2+/Cu is the cathode, where reduction happens. Cu2+ will be reduced to Cu. Equally, Zn2+/Z is the anode, where oxidation happens, Zn will be oxidised to Zn2+.
Anode is written on the left with the Zn(s) and Zn2+(aq) separated by a vertical line as they are in different phases. Cathode is written on the right with the Cu2+(aq) and Cu(s) again separated by a vertical line.
Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
This shows that electrons flow from zinc (which is oxidised) to copper (which is reduced).
Measurement of Electrode Potential
The electrode potential of a half-cell cannot be measured independently. Instead, it measure relative to another half cell.
A reduction potential (also called a standard electrode potential) (E°) tells us how easily the oxidised species in a half-cell gains electrons (is reduced), compared to H+ ions in the standard hydrogen electrode.
Standard conditions:
- 298 K
- 1 mol dm−3 ion concentrations
- 100 kPa for gases
A more positive E° value means a greater tendency for reduction to occur.
A more negative E° value means a greater tendency for oxidation to occur.
Key Point:
When we describe an electrode potential (E°), we are talking about the ease with which the oxidised form of an element or ion in a half cell gains electrons (undergoes reduction).
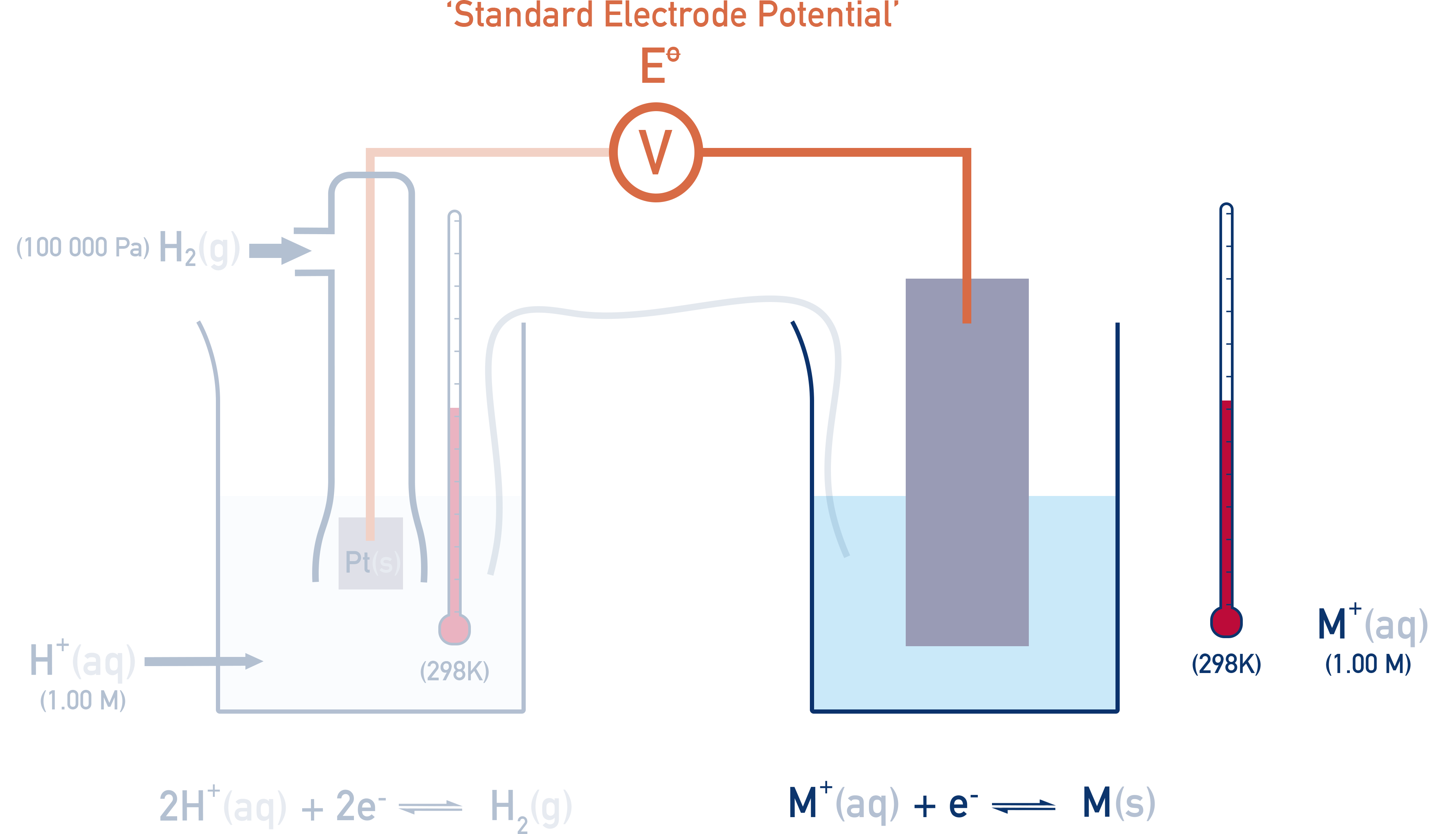
The Standard Hydrogen Electrode (SHE)
The SHE is used as a reference point and is assigned an E° of exactly 0.00V.
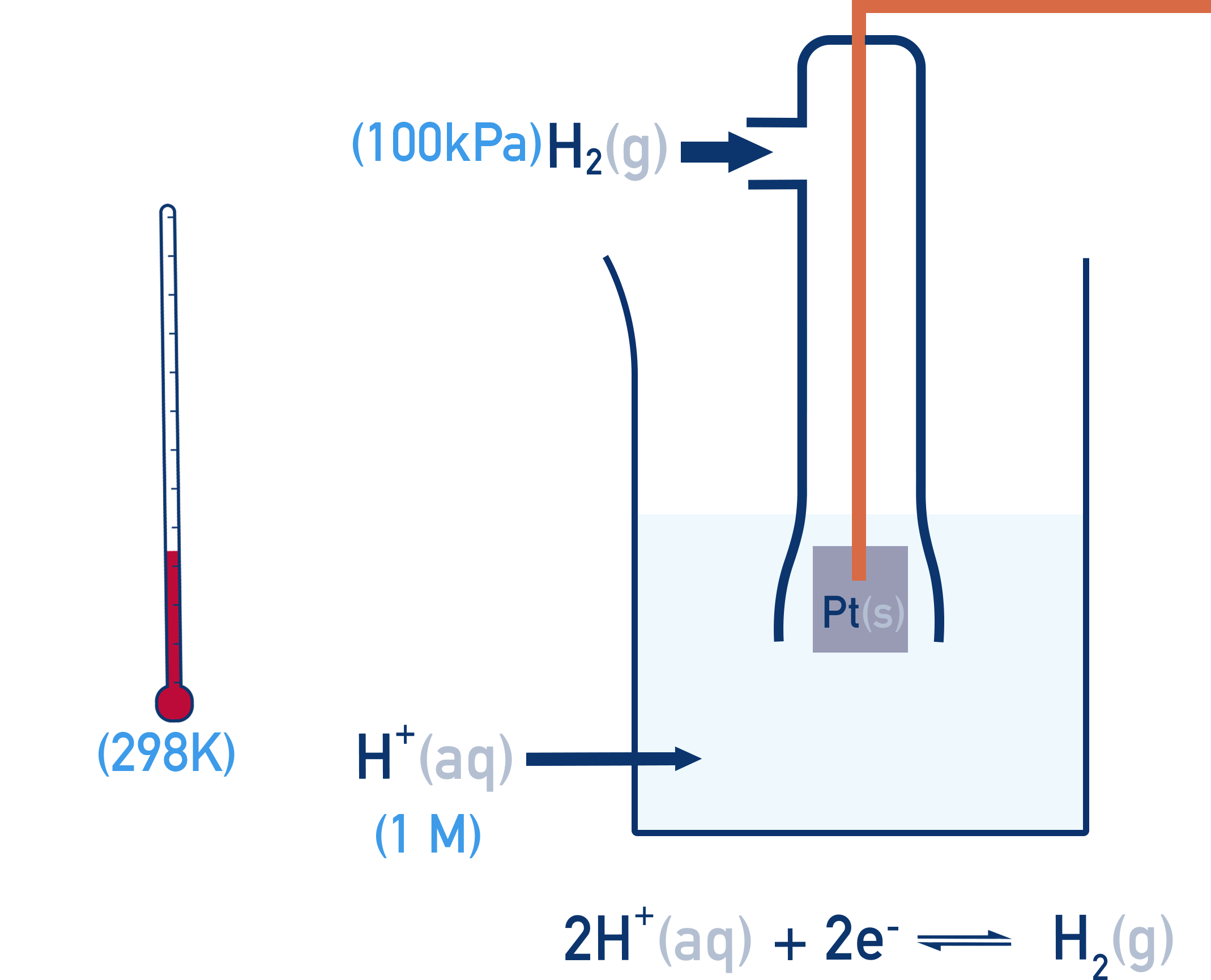
Setup:
- H2 gas at 100 kPa
- 1 mol dm−3 H+ ions (typically from HCl)
- Platinum electrode (inert and conducts electrons)
- 298 K temperature
This means that when two standard hydrogen electrodes are connected together, the potential difference is 0.00V.
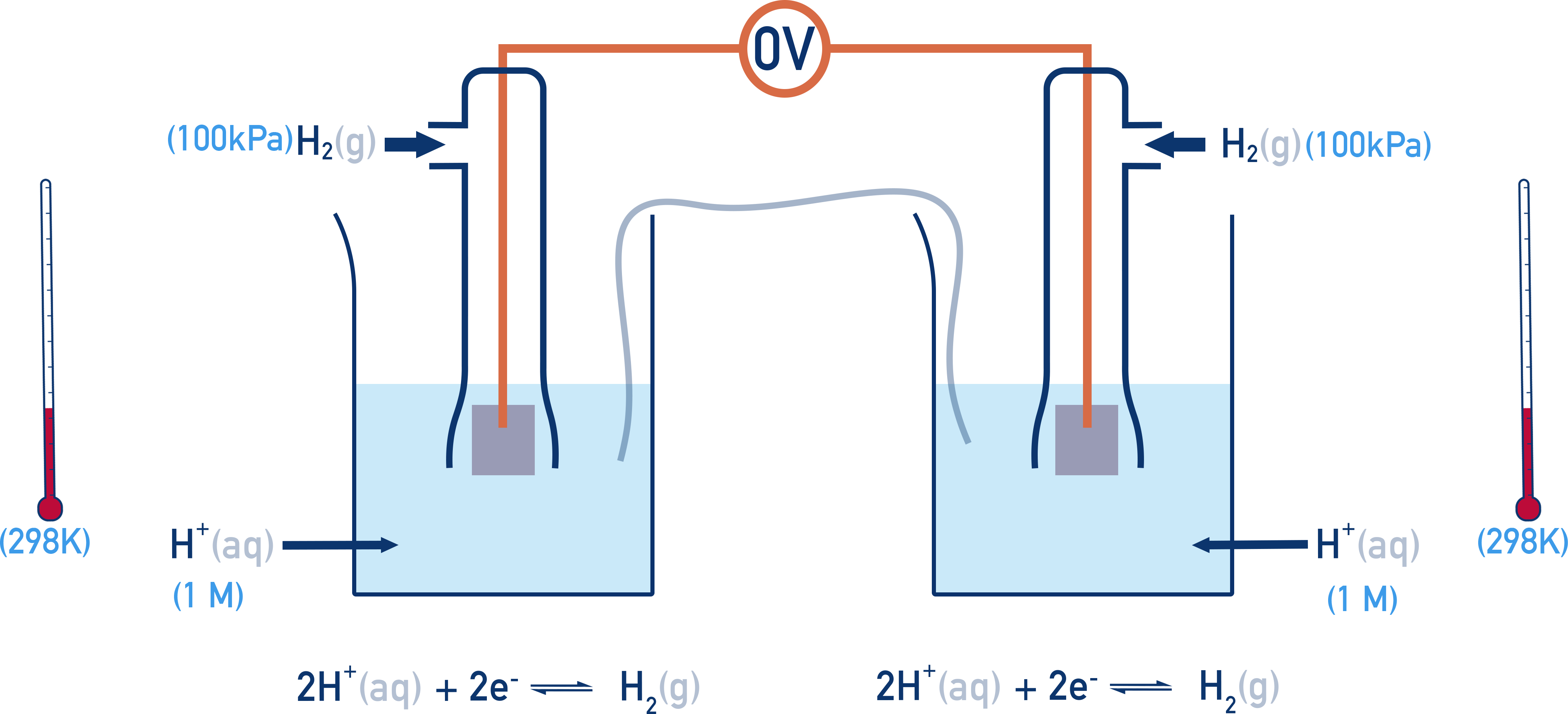
All other standard electrode potentials are measured relative to the SHE. If the right-hand half-cell is now changed, a potential difference (voltage) is measured.
Measuring Reduction Potentials
A half-cell is connected to the SHE and the voltage measured is called the half-cell’s reduction potential, E°.
Reduction potentials are often put into a table called the electrochemical series.
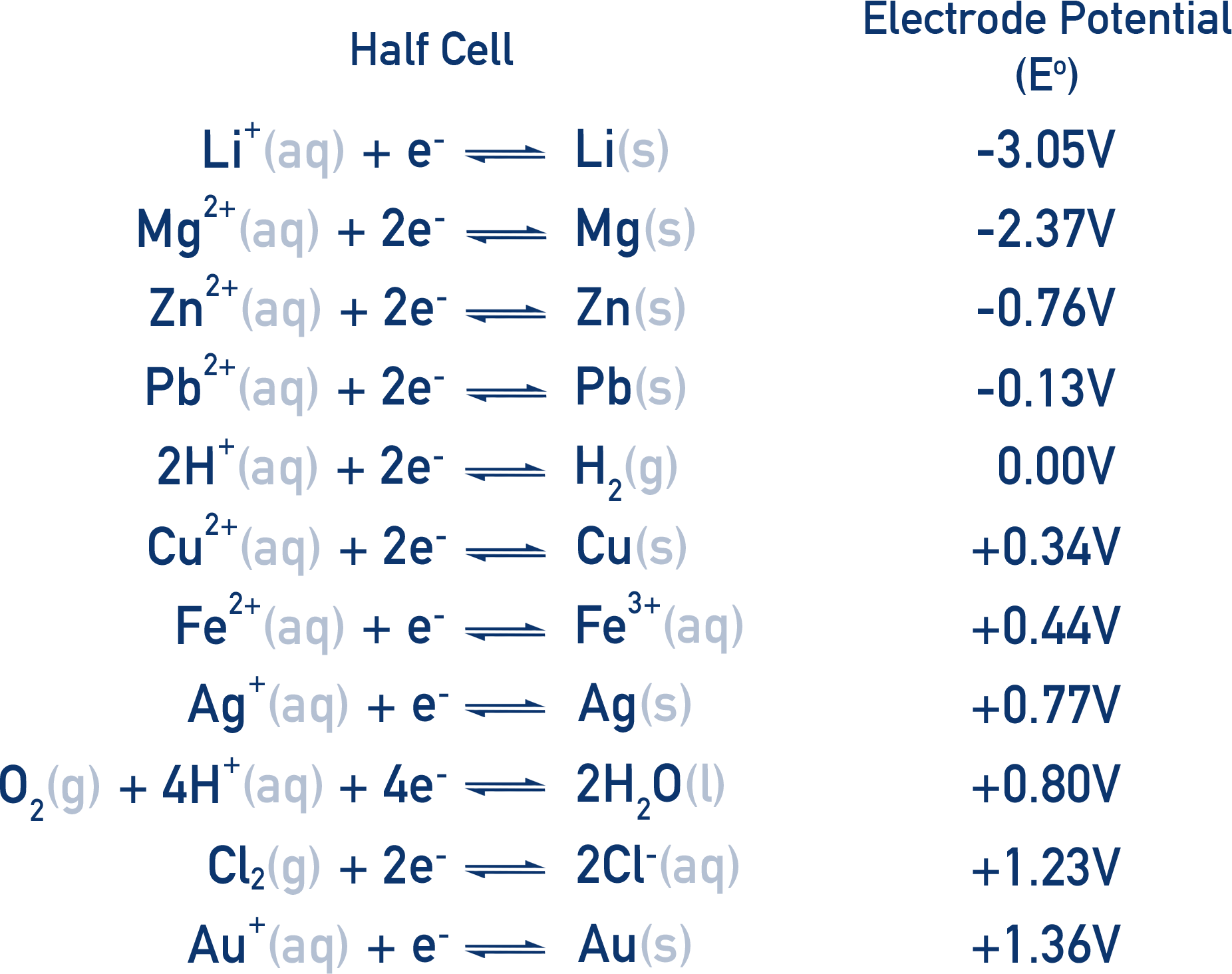
Key Points:
- The more positive the E°, the more likely the species is to be reduced.
- The more negative the E°, the more likely the species is to be oxidised.
- Half-cells can be made in different ways depending on the species involved:
- (a) Metal or Non-Metal Half-Cells
- (b) Different Oxidation States of the Same Element
Calculating and Interpreting E°cell
What Is Standard Cell Potential?
Standard cell potential (E°cell) is the overall potential difference produced by a voltaic cell under standard conditions.
It can be used to tell us whether a redox reaction is spontaneous.
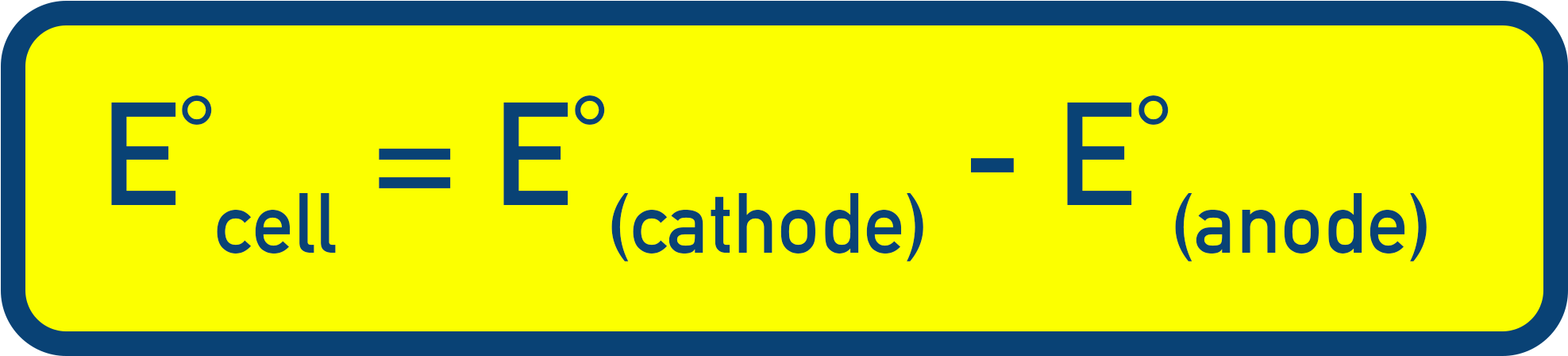
Note:
The cathode is the half-cell where reduction happens (the half-cell with the more positive reduction electrode potential).
The anode is the half-cell where oxidation happens (the half-cell with the more negative reduction potential).
Meaning you can also write this as:


In a spontaneous electrochemical cell, the half-cell with the more positive E° undergoes reduction, and the half-cell with the more negative E° undergoes oxidation. But be careful – in non-spontaneous processes (like electrolysis), this is reversed. Rather than relying on E° signs alone, always check which species is gaining electrons (reduction) and which is losing electrons (oxidation) to avoid mistakes.
Example Zn and Cu Cell
Half-equations and their reduction potentials:
- Zn2+ + 2e− ⇌ Zn (E° = −0.76 V)
- Cu2+ + 2e− ⇌ Cu (E° = +0.34 V)
E°cell = +0.34 V − (−0.76 V) = +1.10 V
→ Spontaneous reaction: Zn + Cu2+ → Zn2+ + Cu
Predicting Spontaneity
- Electron Flow: Electrons flow from the more negative half-cell (anode) to the more positive half-cell (cathode).
- Spontaneous nature or feasibility of process:
- If E°cell is positive, the overall reaction is feasible.
- If E°cell is negative, the reverse reaction is favoured.
- No spontaneity at E°cell = 0 (equilibrium)
Reversibility and Spontaneity
If a redox reaction has a negative E°cell, the forward reaction is not spontaneous.
However, the reverse reaction will be spontaneous, because the electrons would now flow in the opposite direction – from the now more negative to the more positive half-cell.
- If E°cell > 0 → forward reaction is spontaneous
- If E°cell < 0 → reverse reaction is spontaneous
This is useful when predicting whether a proposed redox reaction will proceed as written, or in reverse.
Summary
- Galvanic cells convert chemical energy into electrical energy using spontaneous redox reactions.
- Electrons flow from anode to cathode and the salt bridge maintains electrical neutrality.
- Electrode potentials are measured against the SHE under standard conditions.
- E°cell indicates feasibility and is calculated from cathode and anode E° values.
