Chemical Reactions of Aldehydes and Ketones
Quick Notes
- Reactivity order: Aldehydes > Ketones (due to less steric hindrance and greater electrophilicity).
- Nucleophilic addition is the most common reaction pathway.
- Aldehydes are easily oxidised and ketones resist oxidation.
- Reactions involving α-hydrogens include aldol condensation and haloform reactions.
Full Notes
Aldehydes and ketones exhibit a wide range of reactions due to the polar nature of the carbonyl group. The reactivity is primarily governed by the partial positive charge on the carbonyl carbon, which makes it susceptible to nucleophilic attack.
Nucleophilic Addition Reactions
The carbon in the carbonyl group carries a partial positive charge (δ+) due to the electronegativity of oxygen. This makes it a target for nucleophiles.
The general mechanism involves:

- Nucleophile attacks the electrophilic carbonyl carbon, breaking the π bond.
- The tetrahedral intermediate alkoxide ion is protonated to form the addition product.
Reactivity Order:
Aldehydes are more reactive than ketones because they are less sterically hindered (H vs. R group) and ketones have greater positive inductive (+I) effect from alkyl groups, which reduces the partial positive charge on the carbonyl carbon (δ+).
Key Nucleophilic Addition Reactions
Addition of HCN (Hydrogen Cyanide)
Forms hydroxynitriles (cyanohydrins), important intermediates.
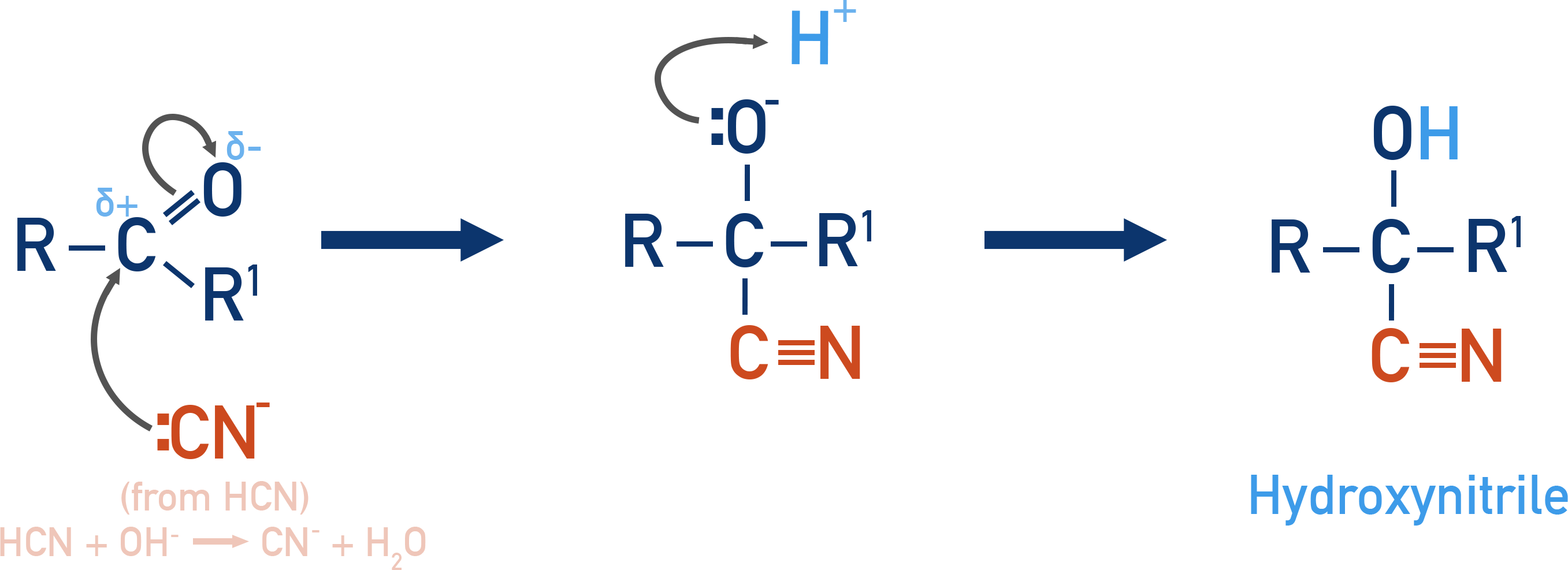
Reagents: HCN (generated in situ from NaCN + dilute HCl)
Example: Formation of a cyanohydrin
CH3CHO + HCN → CH3CH(OH)CN
Addition of Sodium Bisulphite (NaHSO3)
Forms bisulphite addition compounds, which can crystallise and be used for purification.
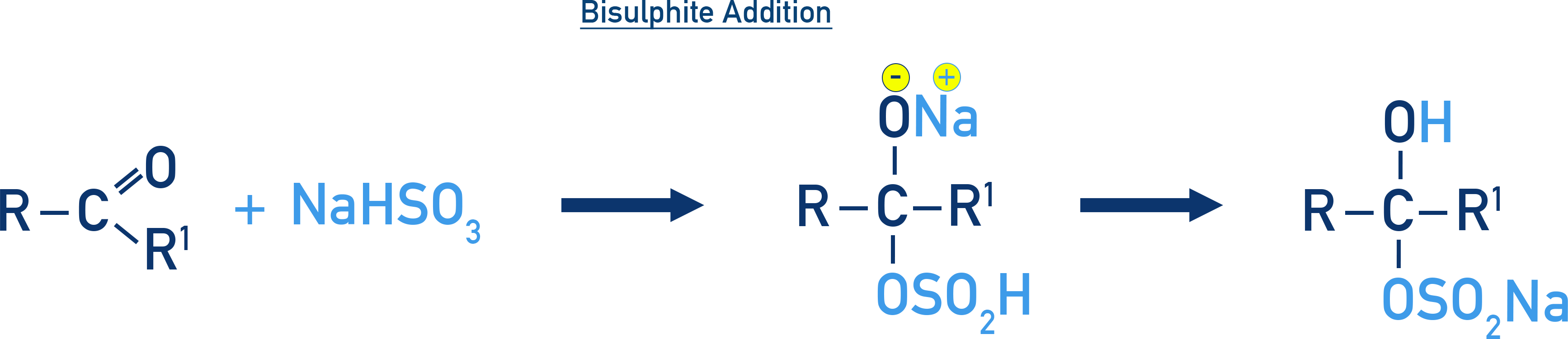
Example: Bisulphite addition product
RCHO + NaHSO3 → RCH(OH)SO3Na
Addition of Alcohols
Reaction with Aldehydes:
Aldehydes react with one equivalent of a monohydric alcohol in the presence of dry HCl gas.
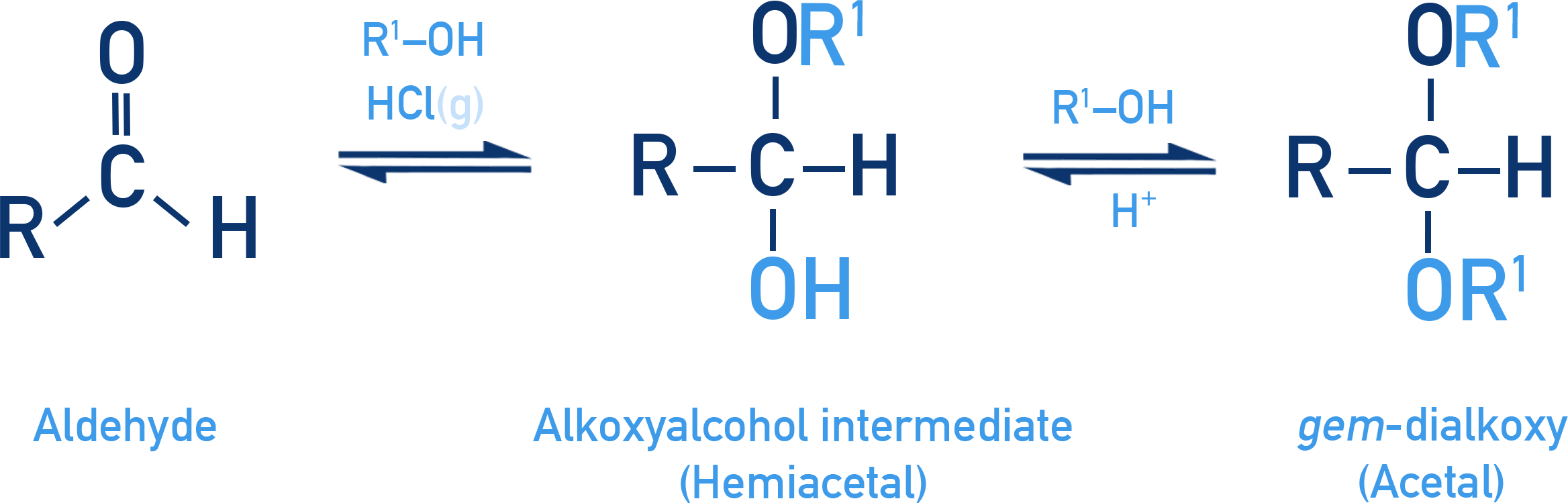
- This forms an intermediate called a hemiacetal:
R–CHO + R'OH ⇌ R–CH(OH)(OR') - The hemiacetal then reacts with another molecule of alcohol to give a gem-dialkoxy compound called an acetal:
R–CH(OH)(OR') + R'OH ⇌ R–CH(OR')2 + H2O
Reaction with Ketones:
Ketones react similarly, but instead of forming acetals, they give ketals. In the presence of ethylene glycol and dry HCl, ketones form cyclic ketals (also called ethylene glycol ketals).
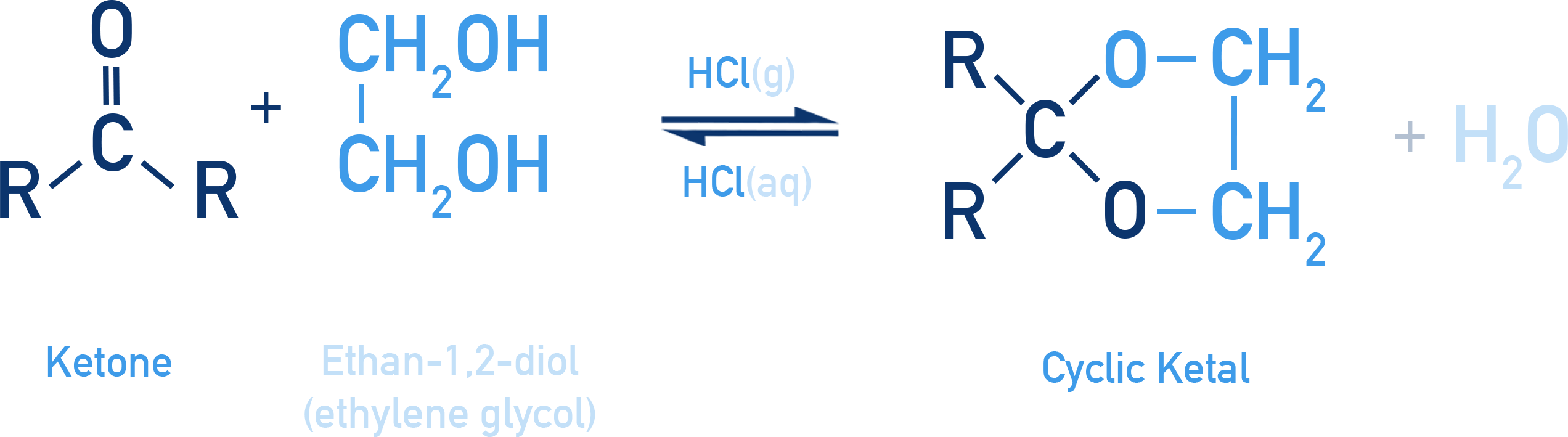
Example: Cyclic ketal formation
R2C=O + HOCH2CH2OH ⇌ R2C(OCH2CH2O) + H2O
Role of Acid Catalyst:
- Dry hydrogen chloride protonates the carbonyl oxygen.
- This increases the electrophilicity of the carbonyl carbon, facilitating nucleophilic attack by the OH group from the alcohol.
- Hydrolysis: Acetals and ketals can be hydrolysed back to the original carbonyl compound using aqueous mineral acids.
Reaction with Ammonia Derivatives
These reactions proceed through the nucleophilic attack of the NH2 group.
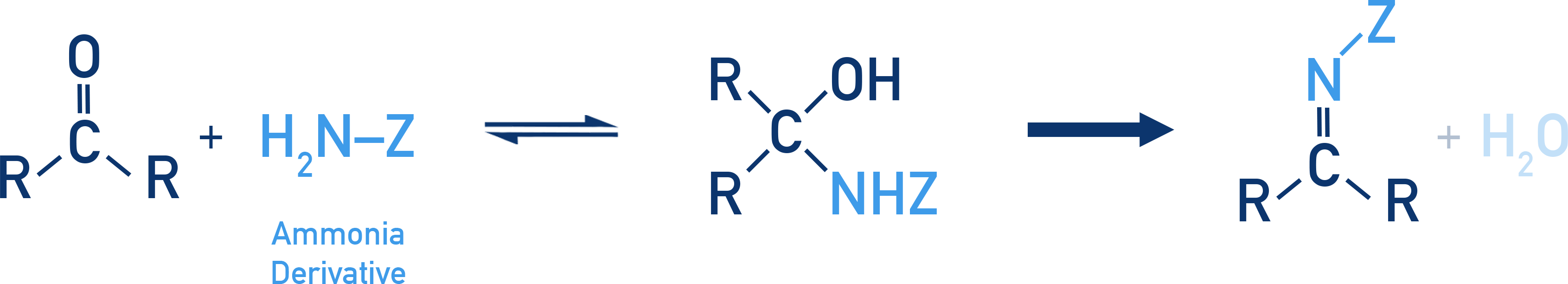
| Reagent | Product | Structure |
|---|---|---|
| NH2OH (hydroxylamine) | Oxime | RCH=NOH |
| NH2NH2 (hydrazine) | Hydrazone | RCH=NNH2 |
| C6H5NHNH2 | Phenylhydrazone | RCH=NNHC6H5 |
| 2,4-Dinitrophenylhydrazine | 2,4-DNP derivative | RCH=NNH–C6H3(NO2)2 |
Again, these reactions are reversible. The derivatives are often used for qualitative identification of carbonyl compounds.
Reduction to Alcohols
Aldehydes and ketones can be reduced to alcohols — aldehydes give primary alcohols, while ketones yield secondary alcohols. This reduction can be carried out using sodium borohydride (NaBH4), lithium aluminium hydride (LiAlH4), or through catalytic hydrogenation.
See Class 12, Unit 7
Reduction to Hydrocarbons
Clemmensen Reduction:
In this method, the carbonyl group (C=O) of aldehydes and ketones is directly reduced to a methylene group (–CH2–).
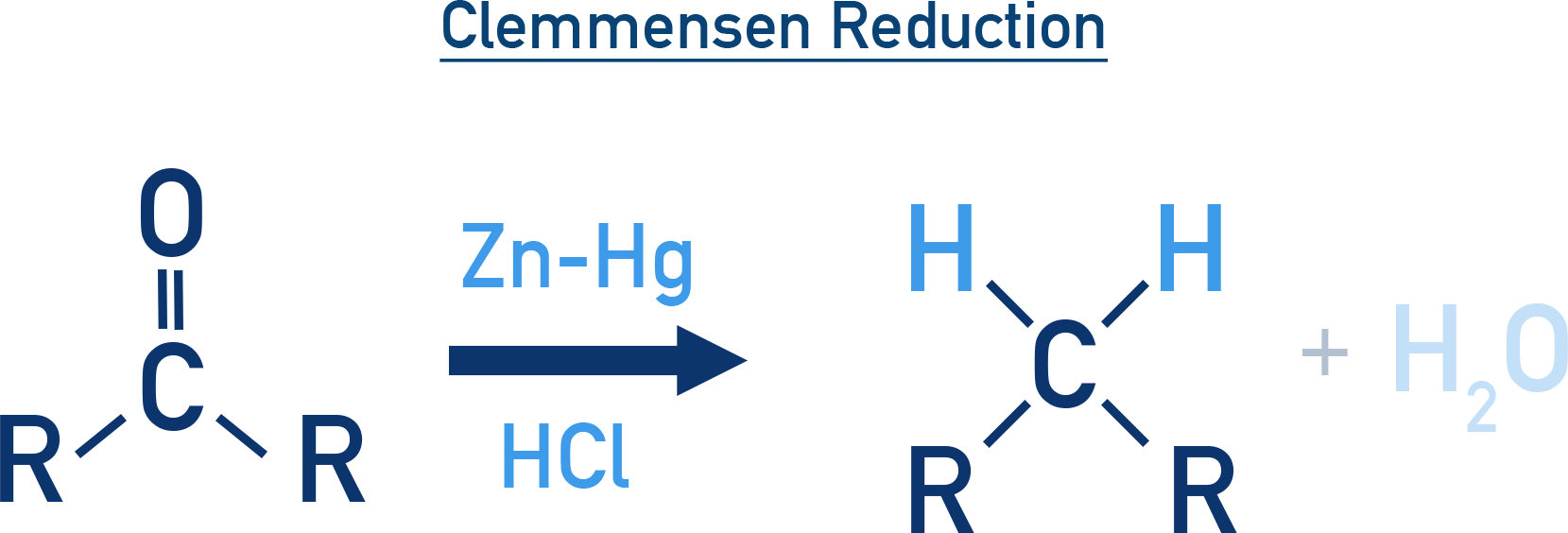
The reaction involves treatment with zinc amalgam (Zn–Hg) and concentrated hydrochloric acid (HCl). This is particularly suitable for compounds stable under strongly acidic conditions.
R–CO–R' → R–CH2–R' Zn–Hg/HCl
Wolff–Kishner Reduction:
This method also reduces the carbonyl group to a methylene group but under strongly basic conditions.
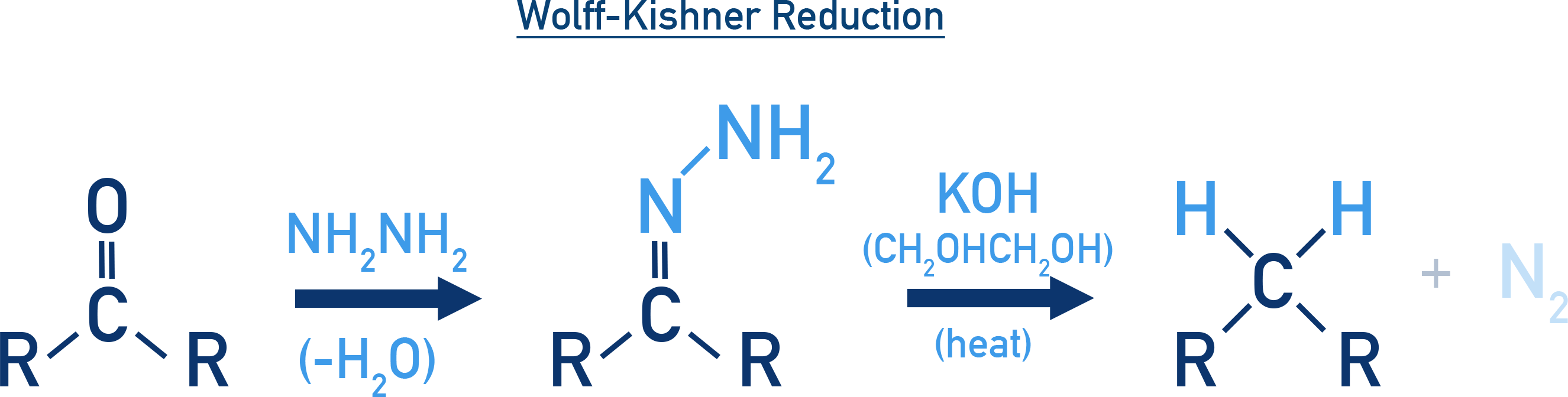
- The compound is first converted to a hydrazone using hydrazine (NH2NH2).
- The hydrazone is then heated with potassium hydroxide (KOH) in a high boiling solvent like ethylene glycol.
- This method is preferred for compounds that are unstable in acidic conditions.
R–CO–R' → R–CH2–R' NH2NH2/KOH, heat
Key Difference:
- Clemmensen Reduction is carried out in acidic medium, while
- Wolff–Kishner Reduction occurs in basic medium.
Oxidation Reactions
Aldehydes
Aldehydes are easily oxidised to carboxylic acids using oxidising agents.
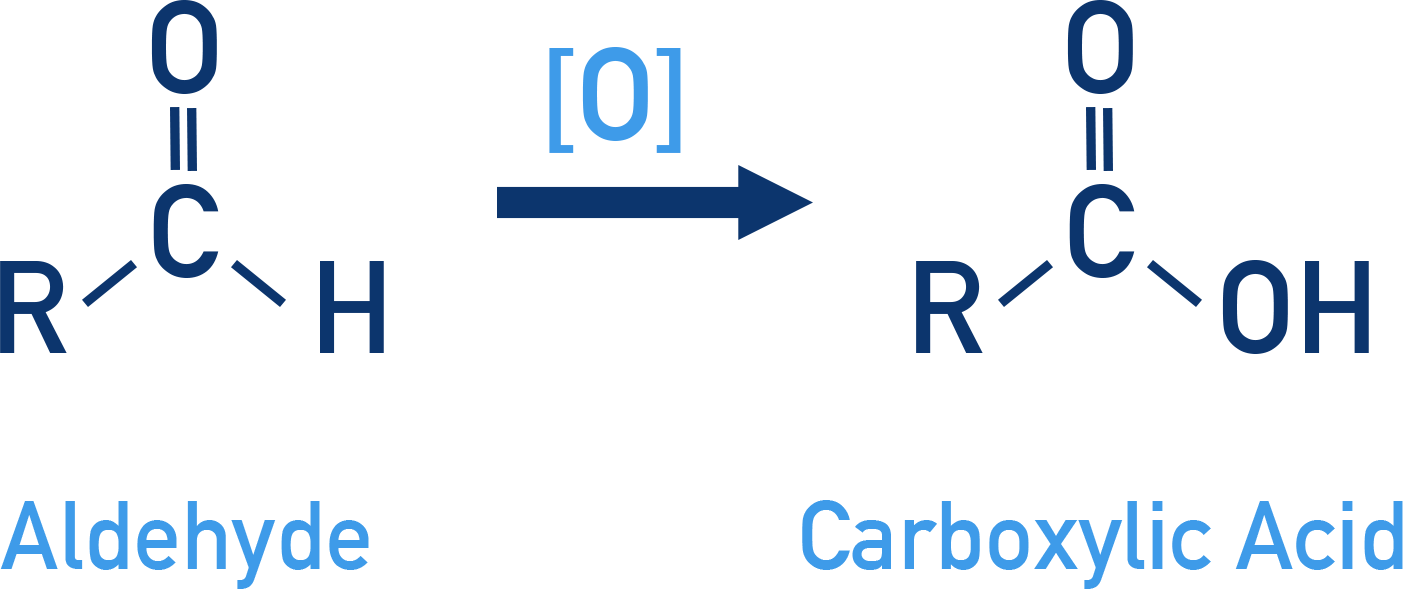
Common oxidising agents, [O], include:
- Nitric acid (HNO3)
- Potassium permanganate (KMnO4)
- Potassium dichromate (K2Cr2O7)
- Even mild oxidants such as Tollens' reagent and Fehling’s reagent
General reaction: R–CHO → R–COOH
Ketones
Ketones are only oxidised under vigorous conditions using strong oxidants and heat.
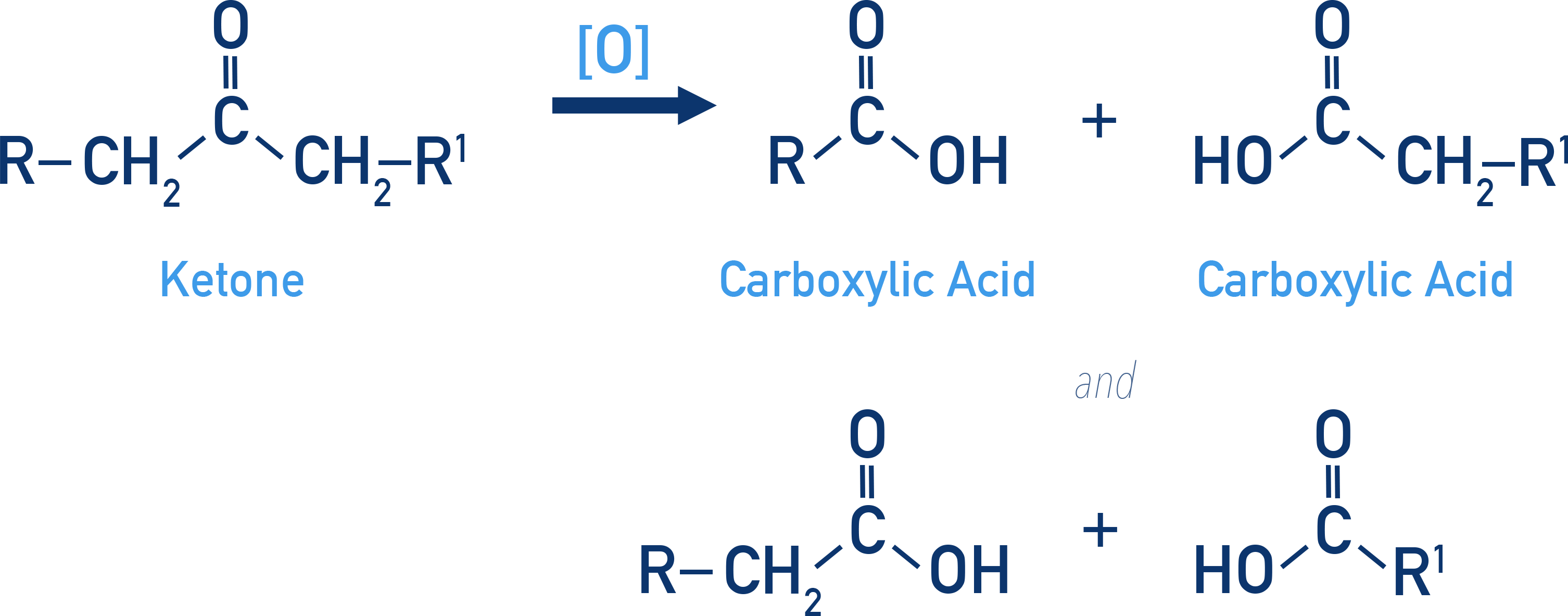
This is because they need to undergo C–C bond cleavage and this produces a mixture of carboxylic acids with fewer carbon atoms than the parent ketone.
Example: Oxidative cleavage
R–CO–CH2–R' → R–COOH + R'–CH2COOH
or
R–CH2COOH + R'–COOH
Distinguishing Aldehydes from Ketones using Mild Oxidants
As aldehydes can be further oxidised, they can be distinguished from ketones (that can’t be further oxidised).
Tollens’ reagent and Fehling’s reactant are commonly used for this purpose.
Tollens’ Test
Reagent: [Ag(NH3)2]+ in NH4OH
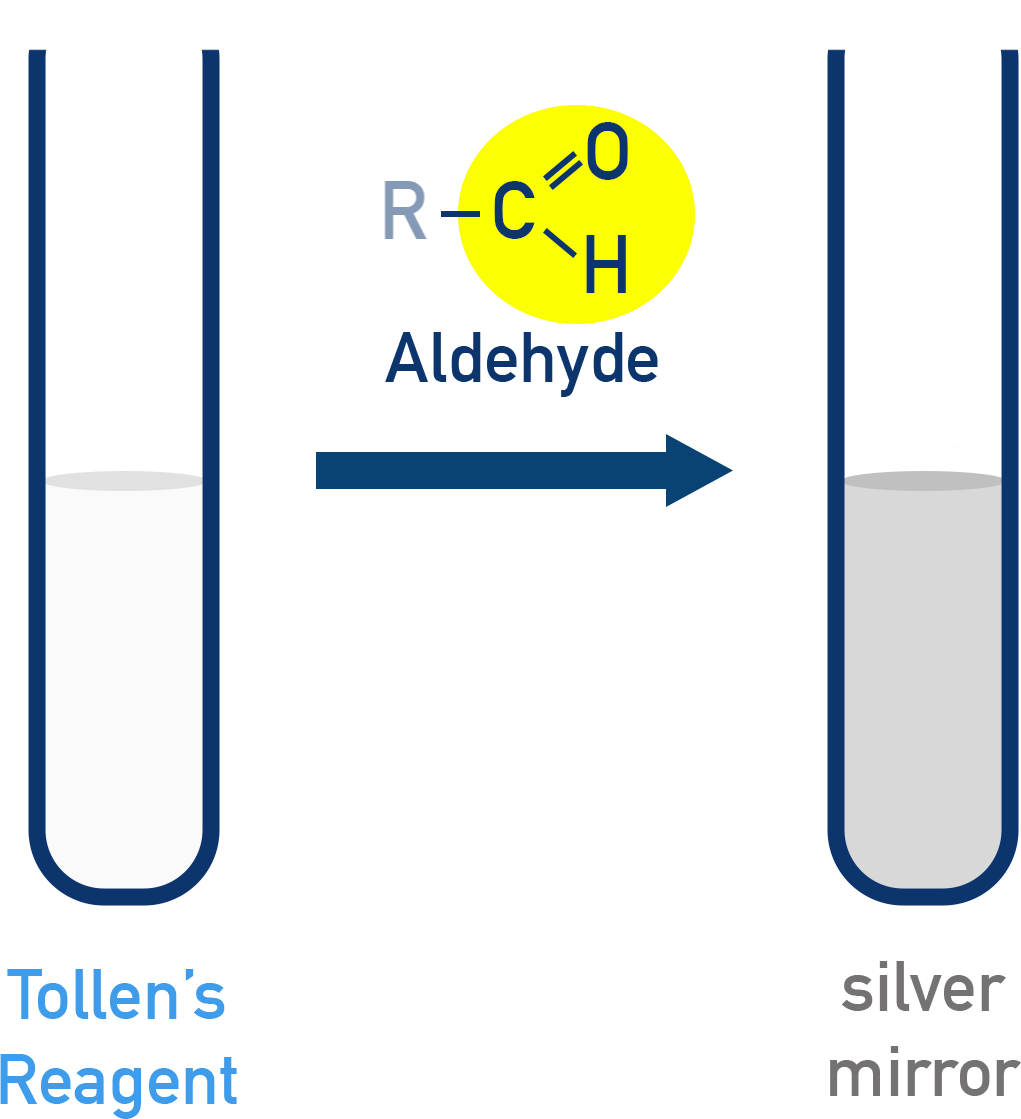
- Aldehyde + Tollens’ → Silver mirror (Ag0 ppt)
- Occurs in alkaline medium
- Aldehyde is oxidised to a carboxylate ion (RCOO−)
Reaction:
R–CHO + 2[Ag(NH3)2]+ + 3OH− → RCOO− + 2Ag + 2H2O + 4NH3
Fehling’s Test
Reagent: Copper(II) sulphate + Rochelle salt (alkaline)
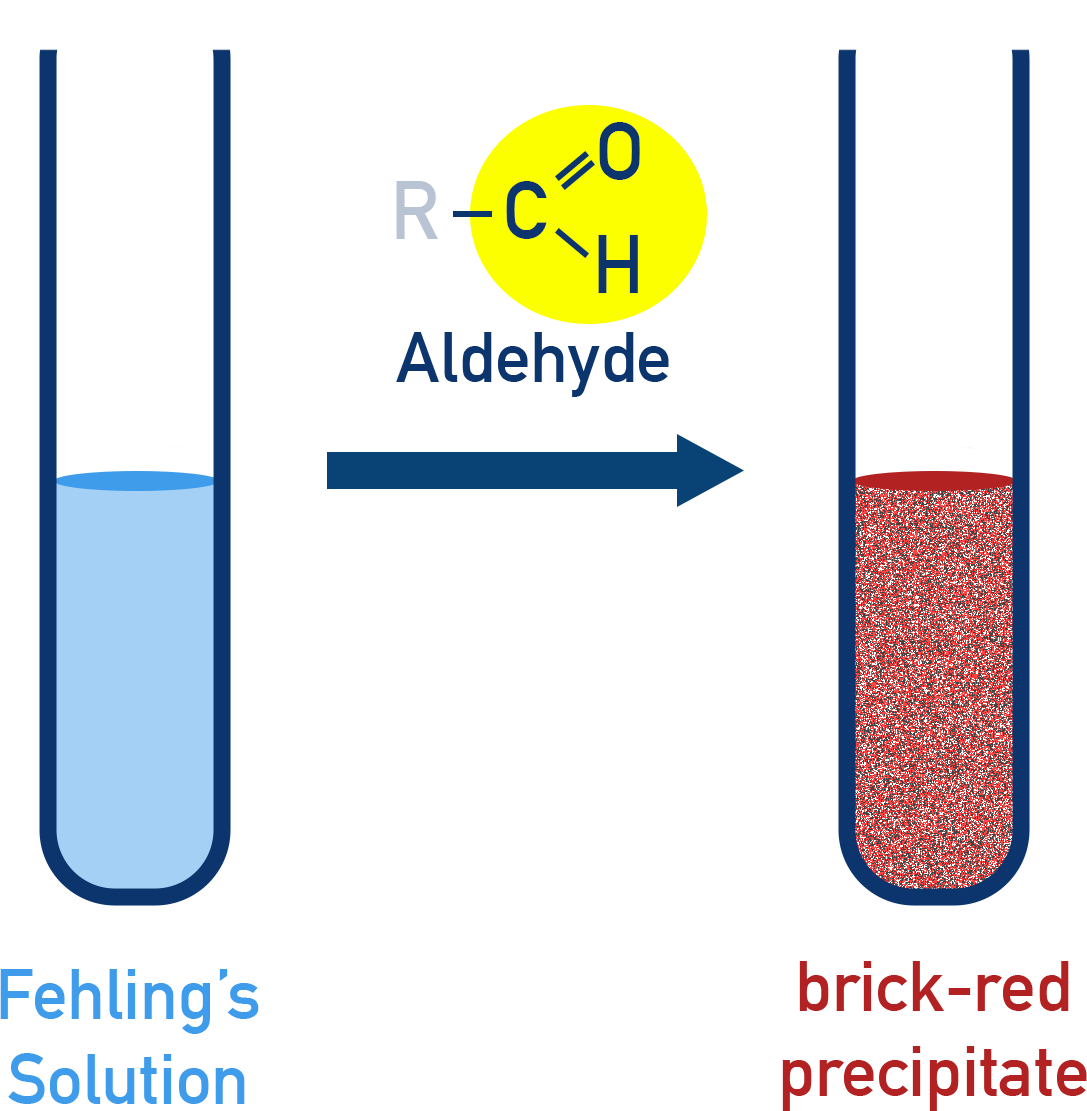
- Positive for aliphatic aldehydes only (aromatic ones like benzaldehyde do not respond)
- Produces reddish-brown Cu2O precipitate
Reaction:
R–CHO + 2Cu2+ + 5OH− → RCOO− + Cu2O + 3H2O
Oxidation of Methyl Ketones: Haloform Reaction
Haloform Test
Aldehydes/ketones with at least one methyl group adjacent to C=O are oxidised by sodium hypohalite (NaOX).
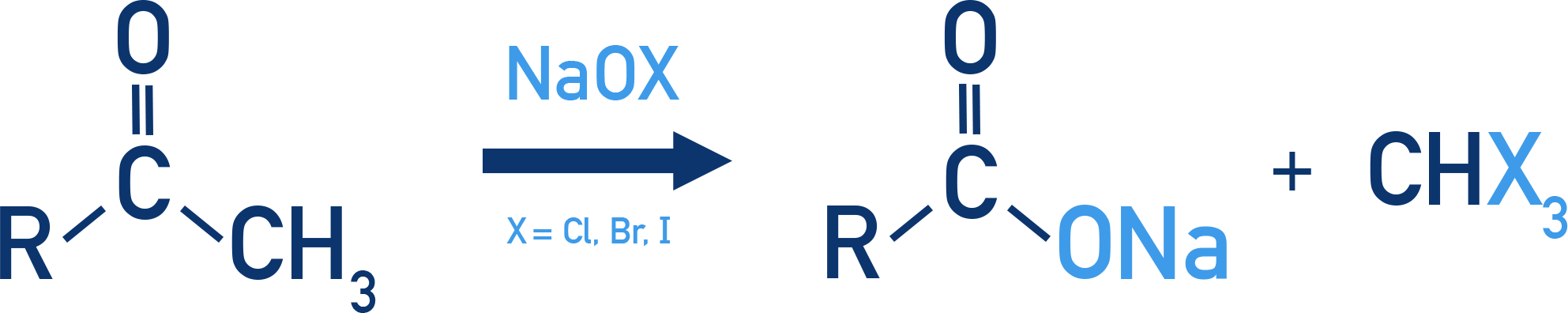
Produces:
- Sodium salt of carboxylic acid (with one less C)
- Haloform (CHX3) like CHCl3
Reagents: NaOCl, NaOBr, NaOI
Example: Haloform formation
R–CO–CH3 + 3NaOX → R–COONa + CHX3
Important feature: Does not oxidise double bonds, only the CH3–CO– group.
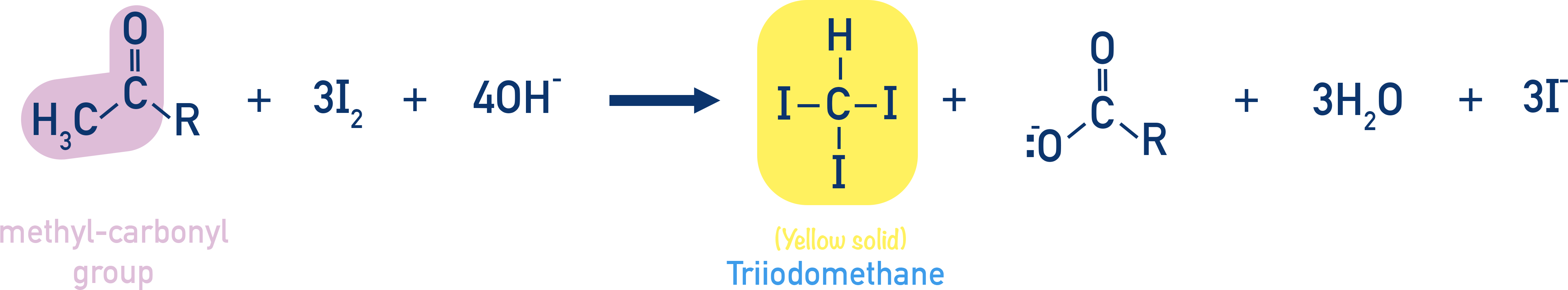
Iodoform reaction with sodium hypoiodite is also used for detection of CH3CO group or CH3CH(OH) group which produces CH3CO group on oxidation.
Reactions Involving α-Hydrogen
α-Hydrogens (attached to the carbon next to the carbonyl group) are acidic. This is because of the electron-withdrawing effect of the carbonyl group and resonance stabilization of the enolate ion formed after deprotonation.
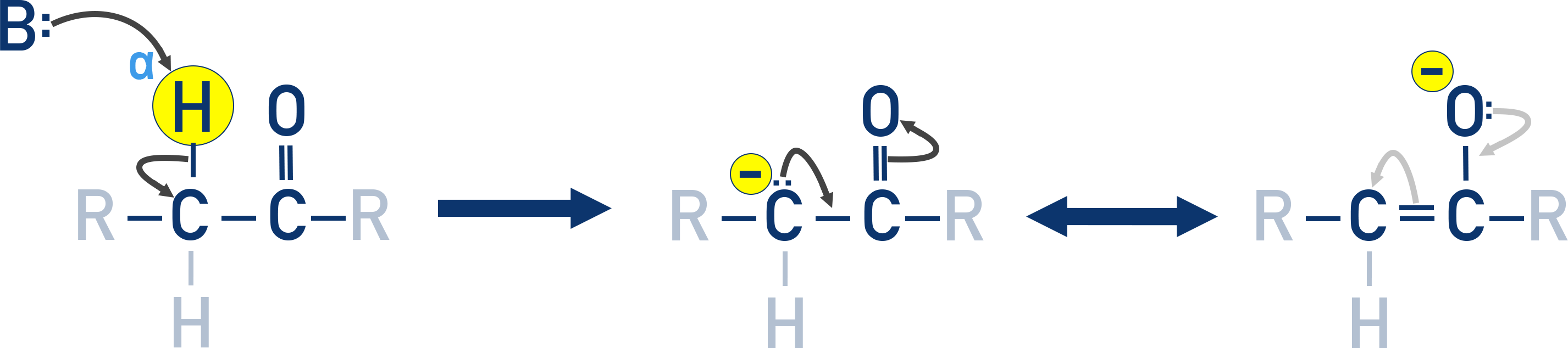
Aldol Condensation
When an aldehyde or ketone has at least one α-hydrogen, it can undergo aldol condensation in the presence of dilute alkali such as NaOH. The reaction proceeds in two steps:
- First, a β-hydroxy aldehyde or β-hydroxy ketone is formed (known as an aldol or ketol).
- On heating, this intermediate undergoes dehydration to yield an α,β-unsaturated carbonyl compound, the aldol condensation product.
Example: Aldol from ethanal
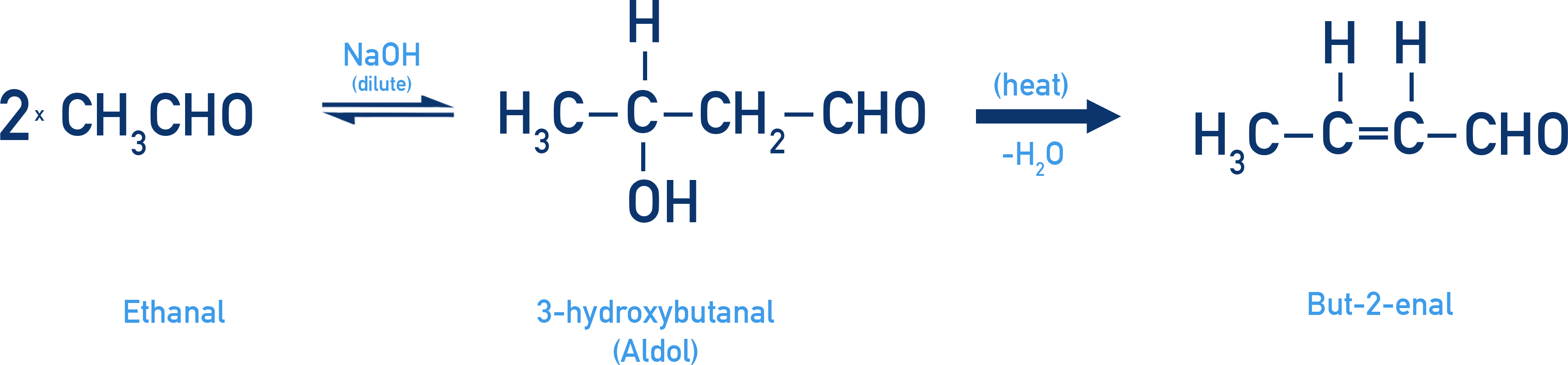
Ethanal (CH3CHO) gives 3-hydroxybutanal as the aldol, which then forms but-2-enal upon dehydration.
Note — The term “aldol” reflects the presence of both aldehyde and alcohol functional groups in the initial product. Even though ketones form ketols, the reaction is still referred to as aldol condensation due to the similar mechanism and outcome.
Cross Aldol Condensation
When the aldol condensation occurs between two different carbonyl compounds (aldehydes and/or ketones), the reaction is known as cross aldol condensation.
If both components contain α-hydrogens, the reaction typically yields a mixture of four products:
- Two from the self-condensation of each reactant.
- Two from cross-condensation between the two different components.
For Example: Ethanal + propanal
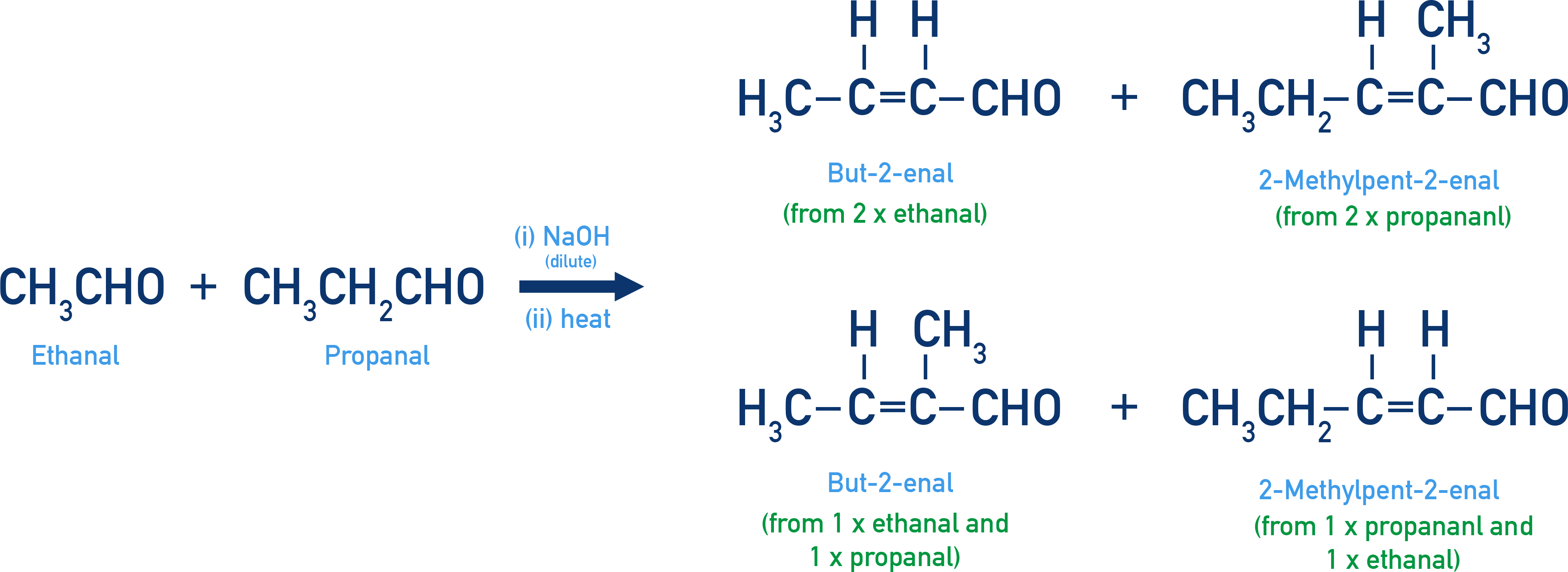
A mixture of ethanal and propanal produces:
- But-2-enal (from two ethanal molecules)
- 2-Methylpent-2-enal (from two propanal molecules)
- 2-Methylbut-2-enal (from ethanal + propanal)
- Pent-2-enal (from propanal + ethanal)
To minimise the number of products, a compound without α-hydrogens (such as benzaldehyde) is often chosen as one of the reactants.
Ketones in Cross Aldol Reactions
Ketones can also act as one component in cross aldol condensations.
For example, benzaldehyde (which lacks α-hydrogens) reacts with acetophenone in the presence of base to form benzalacetophenone (1,3-diphenylprop-2-en-1-one) as the major product.
This strategy helps avoid formation of multiple side products and improves selectivity.
Cannizzaro Reaction
This is a redox reaction unique to aldehydes that lack α-hydrogen atoms, such as formaldehyde or benzaldehyde. When heated with concentrated alkali (e.g., NaOH or KOH), the aldehyde undergoes disproportionation:
- One molecule is oxidised to a carboxylate ion.
- The other is reduced to a primary alcohol.
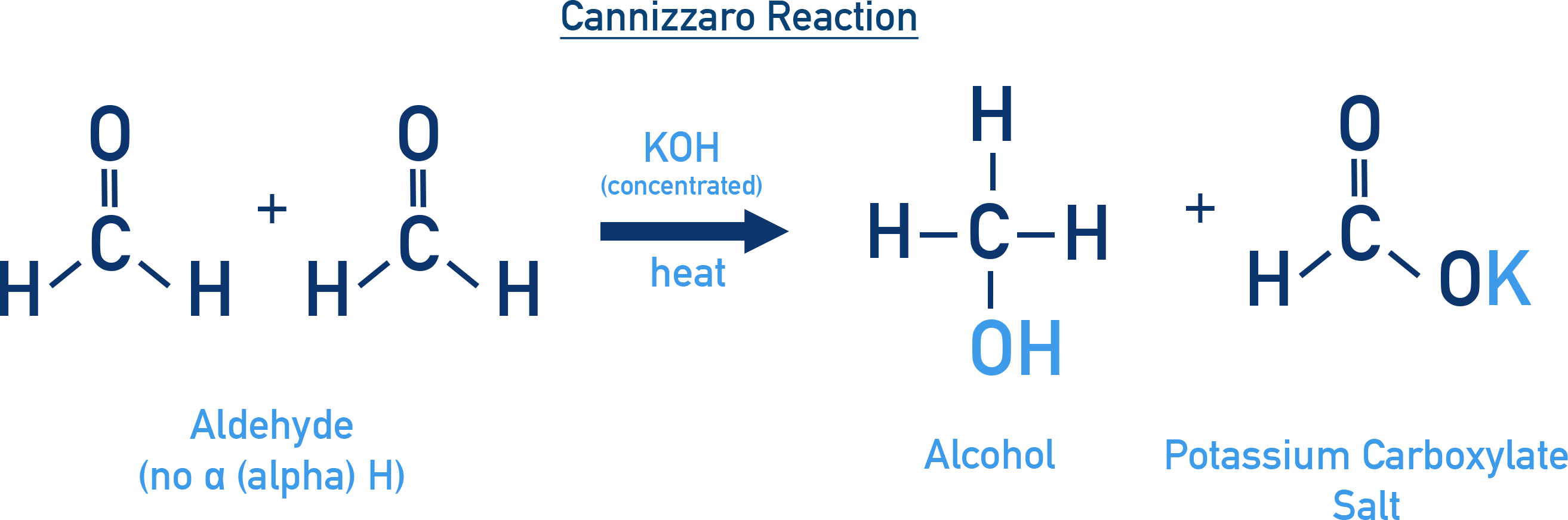
For Example: Formaldehyde
Formaldehyde reacts with conc. KOH to form methanol and potassium formate. This reaction provides a useful method for producing alcohols and acids from aldehydes without using external reducing or oxidising agents.
Electrophilic Substitution in Aromatic Aldehydes
Aromatic aldehydes and ketones can react in typical electrophilic substitution reactions on the benzene ring.
However, the –CHO and –COR groups are:
- Electron-withdrawing, due to the polarity of the carbonyl group.
- Therefore, they are deactivating groups and direct new substituents to the 3rd (meta) position.
For Example: Benzaldehyde nitration
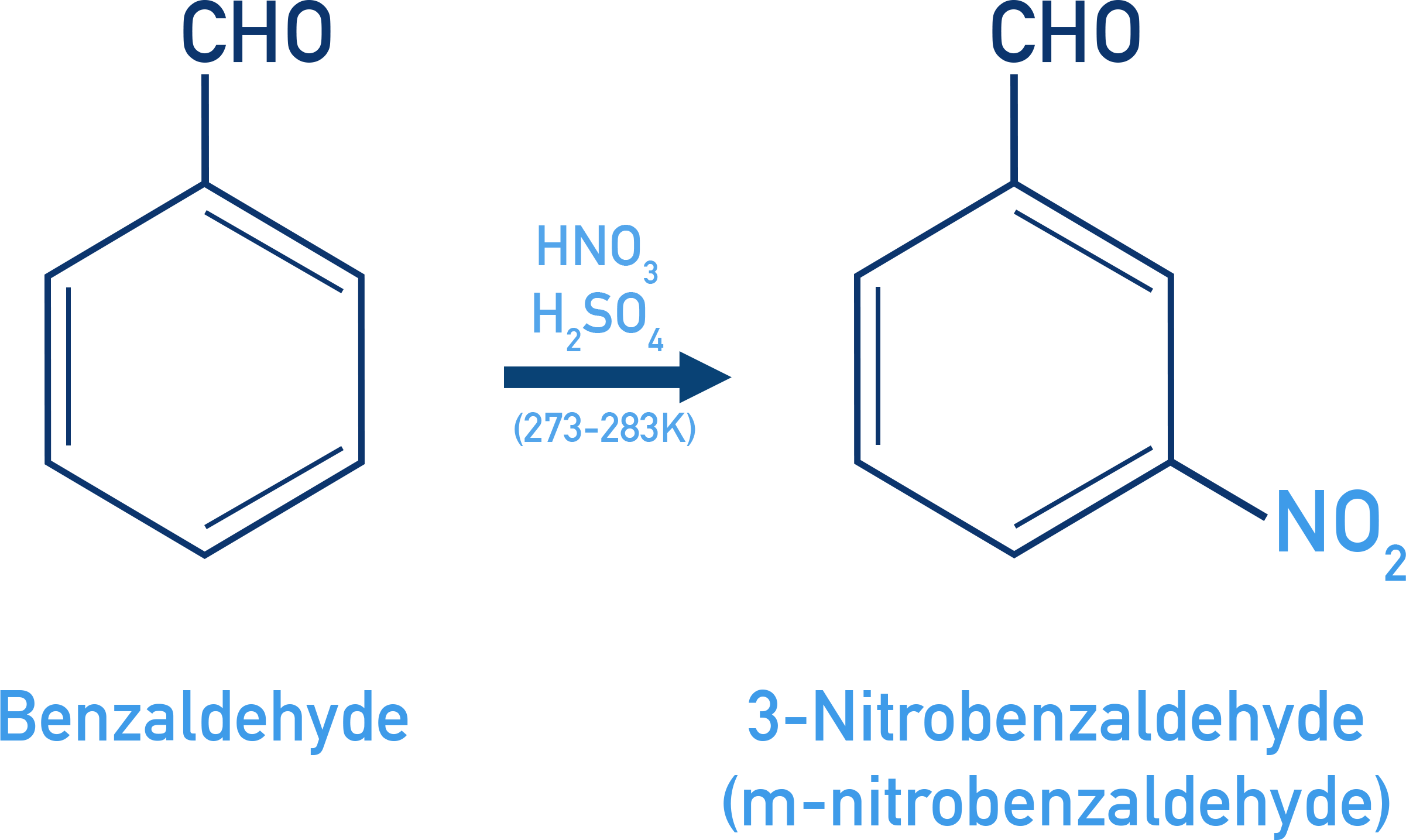
Benzaldehyde reacts with a mixture of concentrated HNO3 and H2SO4 (nitration conditions) to form 3-nitrobenzaldehyde (m-nitrobenzaldehyde) as the major product.
Summary
- Aldehydes are more reactive than ketones in nucleophilic additions due to less steric hindrance and lower +I effects.
- Typical additions include HCN, NaHSO3 and alcohols to form cyanohydrins, bisulphite adducts, acetals and ketals.
- Reductions give alcohols or hydrocarbons via Clemmensen or Wolff–Kishner methods depending on medium.
- Aldehydes oxidise easily while ketones resist and often require cleavage conditions.
- Tollens’ and Fehling’s tests distinguish aldehydes from ketones.
- α-Hydrogen chemistry leads to aldol and haloform reactions and Cannizzaro occurs for non-enolisable aldehydes.
