Bonding in Coordination Compounds
Quick Notes
- Two major theories explain bonding in coordination compounds:
- Valence Bond Theory (VBT) – hybridisation-based
- Crystal Field Theory (CFT) – electrostatic interaction-based
- VBT explains geometry and magnetic properties but not colour or spectral behaviour.
- CFT explains electronic arrangement, colour, and magnetic properties, especially for transition metal complexes.
- The magnitude of crystal field splitting energy (Δ) determines complex type: high-spin or low-spin.
- Both theories have limitations; neither fully explains stability, thermodynamics, or reactivity trends.
Full Notes
Valence Bond Theory
This theory explains the bonding in coordination compounds by considering hybridisation of atomic orbitals in the metal atom or ion. Ligands donate electron pairs into these hybrid orbitals to form coordinate bonds, resulting in specific geometries.
- The central metal ion makes a number of vacant orbitals available for bonding.
- Hybridisation of these orbitals occurs to form equivalent hybrid orbitals.
- Ligands donate lone pairs into these hybrid orbitals, forming coordinate covalent bonds.
- The geometry of the complex is determined by the type of hybridisation:
- sp3 = tetrahedral
- dsp2 = square planar
- d2sp3 = inner orbital octahedral
- sp3d2 = outer orbital octahedral
Examples:
[Co(NH3)6]3+
![NCERT 12 Chemistry VBT example for [Co(NH3)6]3+ showing d2sp3 inner-orbital octahedral hybridisation and electron pairing under NCERT Class 11 board reference.](images/cohybridisation.png)
- Co3+: 3d6 configuration
- Hybridisation: d2sp3 (inner orbital)
- Geometry: Octahedral
- Magnetic behaviour: Diamagnetic (no unpaired electrons)
[CoF6]3−
![NCERT 12 Chemistry VBT example for [CoF6]3− showing sp3d2 outer-orbital octahedral hybridisation with four unpaired electrons under NCERT Class 11 reference.](images/coparamagnetic.png)
- Co3+: 3d6 configuration
- F− is a weak field ligand → no pairing
- Hybridisation: sp3d2 (outer orbital)
- Geometry: Octahedral
- Magnetic behaviour: Paramagnetic (4 unpaired electrons)
[NiCl4]2−
![NCERT 12 Chemistry VBT example for [NiCl4]2− showing sp3 tetrahedral hybridisation with two unpaired electrons per NCERT Class 12 syllabus.](images/niparamagnetic.png)
- Ni2+: 3d8 electronic configuration
- Cl− is a weak field ligand → no pairing of electrons
- Hybridisation: sp3 (outer orbital hybridisation)
- Geometry: Tetrahedral
- Magnetic behaviour: Paramagnetic (with 2 unpaired electrons)
[Ni(CN)4]2−
![NCERT 12 Chemistry VBT example for [Ni(CN)4]2− showing dsp2 square planar hybridisation and diamagnetism consistent with NCERT Class 12 content.](images/nidiamagnetic.png)
- Ni2+: 3d8 electronic configuration
- CN− is a strong field ligand → pairing occurs
- Hybridisation: dsp2 (inner orbital hybridisation)
- Geometry: Square planar
- Magnetic behaviour: Diamagnetic (no unpaired electrons)
Magnetic Properties of Coordination Compounds
The magnetic behaviour of a coordination compound depends on the presence or absence of unpaired electrons in its d-orbitals. This can be measured experimentally and helps in predicting the electronic configuration and nature of the complex.
d-Electron Configurations and Hybridisation:
- For metal ions with up to 3 electrons in d orbitals (e.g. Ti3+, V3+, Cr3+ → d1, d2, d3):
- Two vacant d-orbitals are available for octahedral hybridisation (with 4s and 4p orbitals).
- Their magnetic behaviour is consistent whether free or in complexes.
- When more than 3 d-electrons are present:
- Hund’s rule restricts pairing, making inner d-orbital hybridisation less accessible.
- For d4 (e.g. Cr2+, Mn3+), d5 (e.g. Mn2+, Fe3+), d6 (e.g. Fe2+, Co3+): pairing for inner orbital hybridisation is difficult, leading to outer orbital hybridisation instead.
| Compound | Observed Behaviour | Explanation |
|---|---|---|
| [Mn(CN)6]3− | 2 unpaired electrons | Low-spin, inner orbital complex |
| [MnCl6]3− | 4 unpaired electrons | High-spin, outer orbital complex |
| [Fe(CN)6]3− | 1 unpaired electron | Low-spin, inner orbital |
| [FeF6]3− | 5 unpaired electrons | High-spin, outer orbital |
| [CoF6]3− | 4 unpaired electrons | Outer orbital, high-spin |
| [Co(C2O4)3]3− | Diamagnetic | Inner orbital, low-spin |
Limitations of Valence Bond Theory
Although valence bond theory (VBT) explains bonding and magnetic properties to some extent, it has several limitations that restrict its use with more advanced coordination chemistry.
- It does not explain the colour of complexes.
- It provides no insight into the relative strengths of ligands.
- It does not distinguish between high-spin and low-spin complexes.
- It cannot predict the spectra of complexes.
- It ignores the quantitative aspects of bonding and thermodynamic stability.
Crystal Field Theory
Crystal Field Theory models metal-ligand bonding as purely electrostatic, explaining how the presence of ligands causes splitting of d-orbitals. It provides a better understanding of the geometry, colour, and magnetism of complexes.
- Ligands are treated as point charges (if anionic) or dipoles (if neutral molecules).
- In a spherical field of negative charge, all five degenerate d-orbitals of a free metal ion experience equal repulsion from the surrounding negative field, resulting in a uniform increase in their energy.
- However, in the presence of directional fields—such as octahedral, tetrahedral, or square planar geometries—the d-orbitals experience varying degrees of repulsion depending on their spatial orientation.
- Because each d-orbital is shaped differently, some interact more strongly with the ligand field than others. This causes the previously degenerate (equal-energy) d-orbitals to split into two distinct sets: one with higher energy and one with lower energy.
- When ligands coordinate to a central metal ion, this crystal field effect causes the d-orbitals to split based on the geometry and strength of the ligand field.
The extent of crystal field splitting (Δo) depends on two main factors:
- The nature of the ligand (i.e., how strong or weak its field is), and
- The charge on the metal ion (higher charge → greater splitting).
Some ligands produce a strong field, causing a large energy gap between the split d-orbitals. Others produce a weaker field, leading to smaller splitting.
The spectrochemical series is an experimentally determined ranking of ligands from weakest to strongest field strength based on how much they split the d-orbitals in metal complexes.
Example:
I− < Br− < SCN− < Cl− < F− < OH− < H2O < NH3 < en < CN− < CO
Octahedral Field Splitting
Five d-orbitals split into:
- t2g (dxy, dyz, dxz) = lower energy
- eg (dz2, dx2−y2) = higher energy
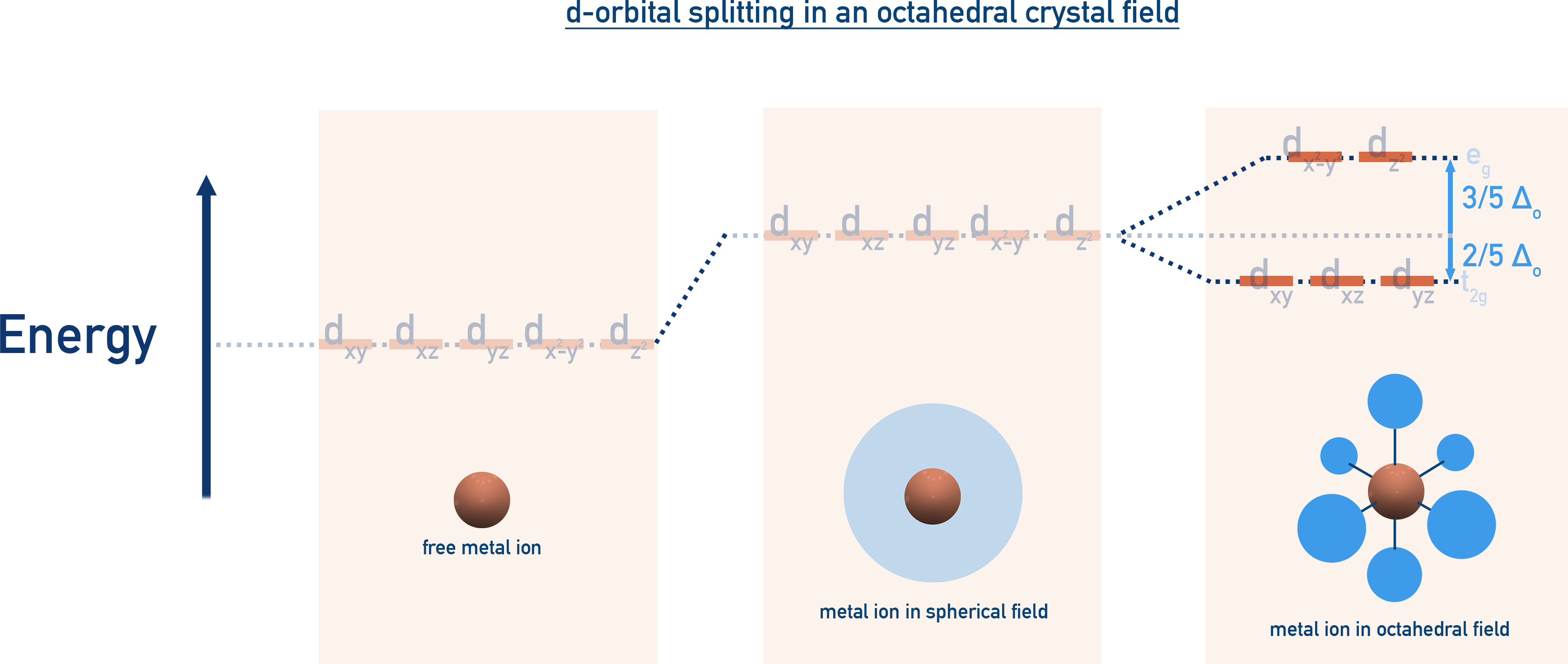
- The energy difference is called crystal field splitting energy (Δo).
- Strong field ligands (e.g., CN−) give large Δo and pairing of electrons in orbitals occurs = low-spin complex
- Weak field ligands (e.g., F−) give small Δo and pairing of electrons in orbitals does not occur = high-spin complex
Tetrahedral Field Splitting
Reverse splitting:
- t2 (dx2−y2, dz2) = lower energy
- e (dxy, dyz, dxz) = higher energy
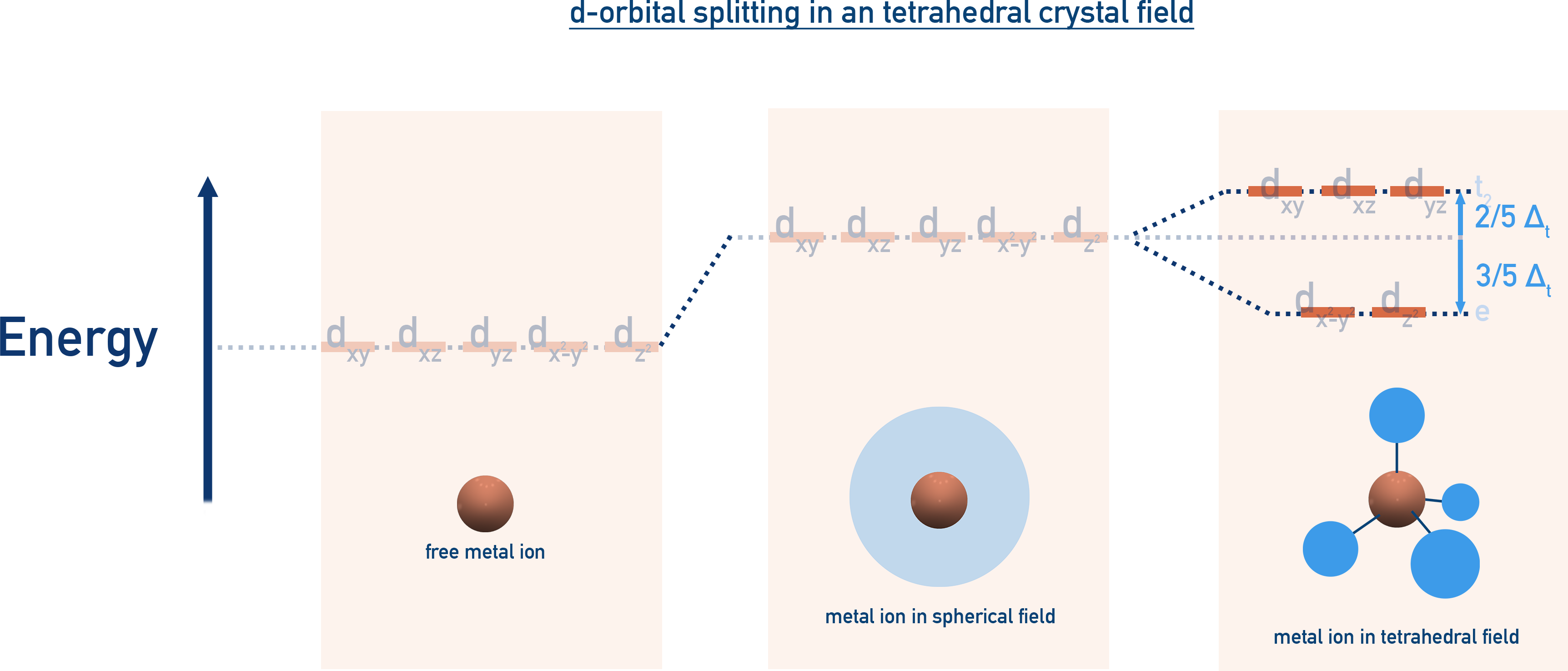
- Crystal field splitting (Δt) is smaller than Δo, so high-spin complexes are favoured.
Crystal Field Stabilisation Energy (CFSE): Difference in energy between the d-orbital configuration in free ion vs complex. Explains stability and colour trends.
Colour in Coordination Compounds
The colours of coordination compounds are due to electronic transitions within the d-orbitals.
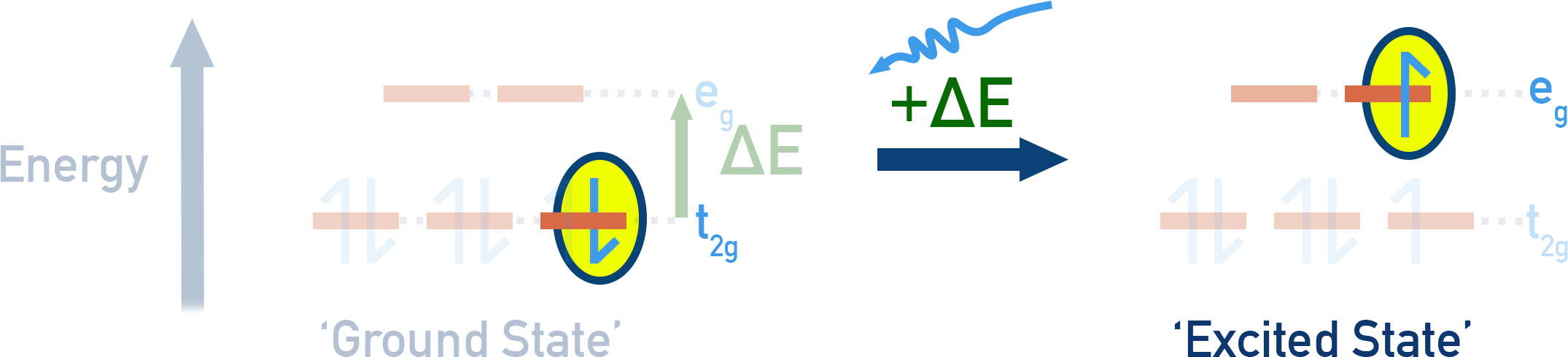
When visible light is absorbed to promote electrons between split d-levels, the complementary colour is observed.
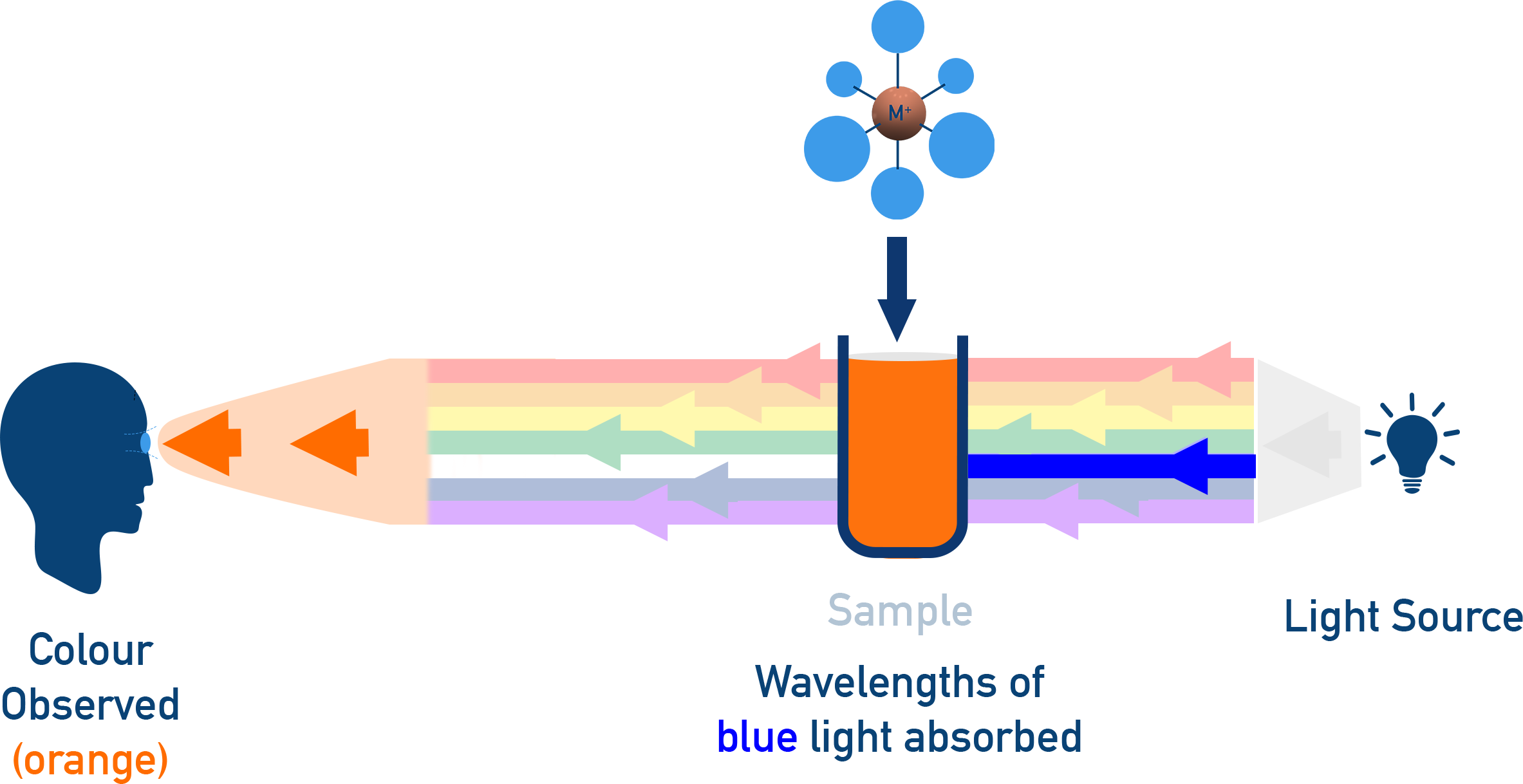
- Most coordination compounds are coloured due to d–d transitions.
- When white light strikes a complex:
- One wavelength is absorbed (corresponding to electronic transition between d-orbitals)
- The complementary colour is observed
- Example: [Ti(H2O)6]3+ appears purple due to absorption in the green-yellow region.
Note: If all d-electrons are paired, or if no d-electrons are present, the complex is colourless.
Limitations of Crystal Field Theory
- It assumes purely electrostatic interaction, ignoring covalent character in bonding.
- It cannot explain the spectral and thermodynamic properties of all complexes.
- It overestimates the ionic nature of metal-ligand bonds.
- Fails to explain the ligand field strength order (spectrochemical series) in terms of bonding.
Summary
- Valence Bond Theory describes hybridisation and predicts geometry and magnetism.
- Crystal Field Theory explains d-orbital splitting, colour and spin states.
- Strong field ligands give large splitting and often low-spin complexes.
- Weak field ligands give small splitting and often high-spin complexes.
- Both VBT and CFT have limitations for stability and reactivity trends.
