Alcohols and Phenols
Quick Notes
- Alcohols are prepared from alkenes, carbonyl compounds, and Grignard reagents.
- Phenols are prepared from haloarenes, benzenesulphonic acid, diazonium salts, and cumene.
- Boiling points increase with hydrogen bonding and molecular mass.
- Solubility depends on the number of –OH groups and chain length.
- Reactions of alcohols and phenols involve:
- Cleavage of O–H bond (acidity, esterification)
- Cleavage of C–O bond (halide substitution, dehydration, oxidation)
- Electrophilic substitution in phenols (e.g. nitration, halogenation)
- Special reactions: Kolbe’s, Reimer-Tiemann, oxidation, zinc dust
Full Notes
Alcohols can be synthesized through multiple pathways involving addition, reduction, and organometallic reagents.
Alcohols From Alkenes
Water is added across the double bond to form alcohols.
Acid-Catalysed Hydration
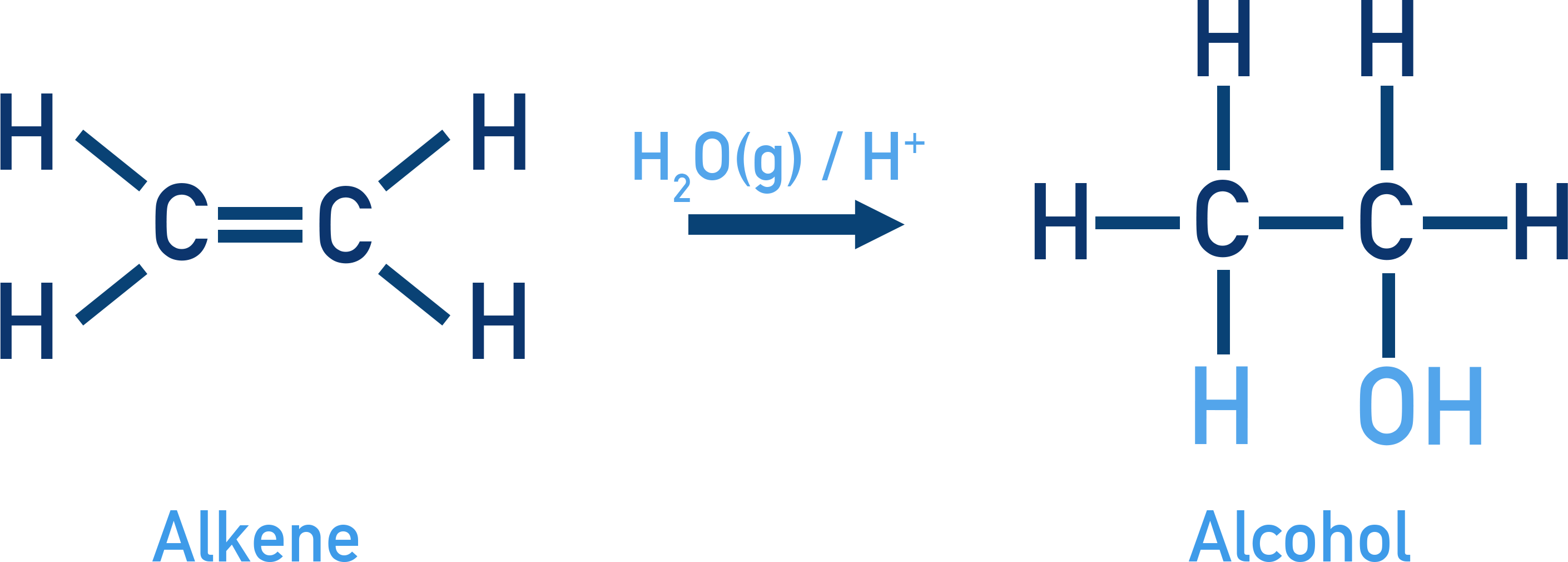
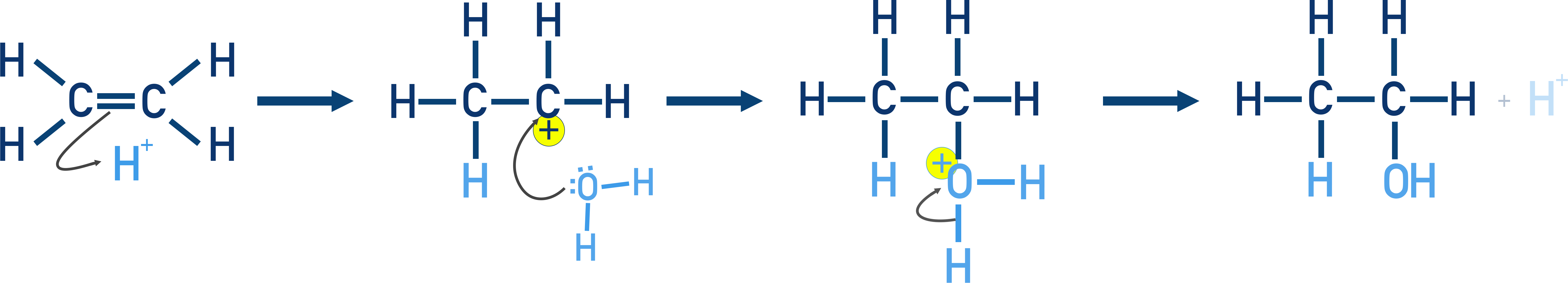
Water adds to alkenes in the presence of dilute H2SO4 via carbocation intermediates.
Follows an electrophilic addition mechanism and Markovnikov's rule.
Mechanism:
Example Hydration of propene
CH3–CH=CH2 + H2O (H+) → CH3–CH(OH)–CH3
Hydroboration–Oxidation
A two-step reaction that avoids rearrangement and gives anti-Markovnikov product.
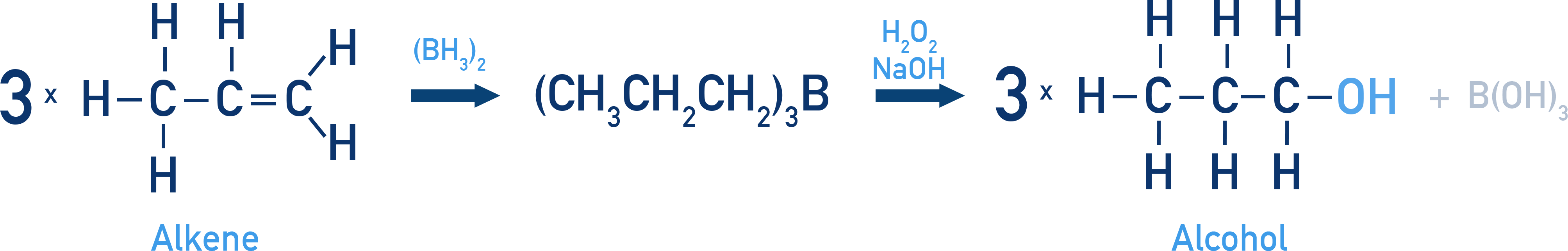
Uses BH3•THF followed by H2O2/NaOH.
Example Ethene to ethanol
CH2=CH2 → CH3–CH2OH
Alcohols From Carbonyl Compounds
Carbonyl groups undergo reduction to yield alcohols.
Reduction using NaBH4 or LiAlH4
Aldehydes form primary alcohols and ketones form secondary alcohols.
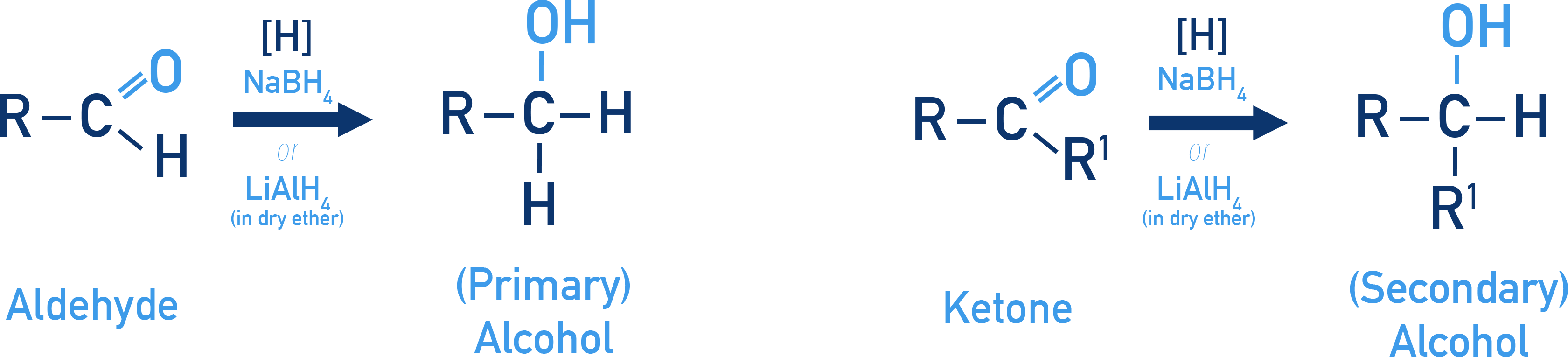
LiAlH4 is a more powerful reducing agent than NaBH4, because of this if LiAlH4 is used and no water can be present and the reaction must be carried out in dry ether.
Examples Typical reductions
- CH3CHO → CH3CH2OH
- CH3COCH3 → CH3CH(OH)CH3

Remember reduction in organic chemistry is the gaining of a carbon-hydrogen bond. To provide the hydrogen needed, we use reducing agents (such as NaBH4 and LiAlH4) and show hydrogen from a reducing agent in equations as [H].
Reduction of Carboxylic Acids
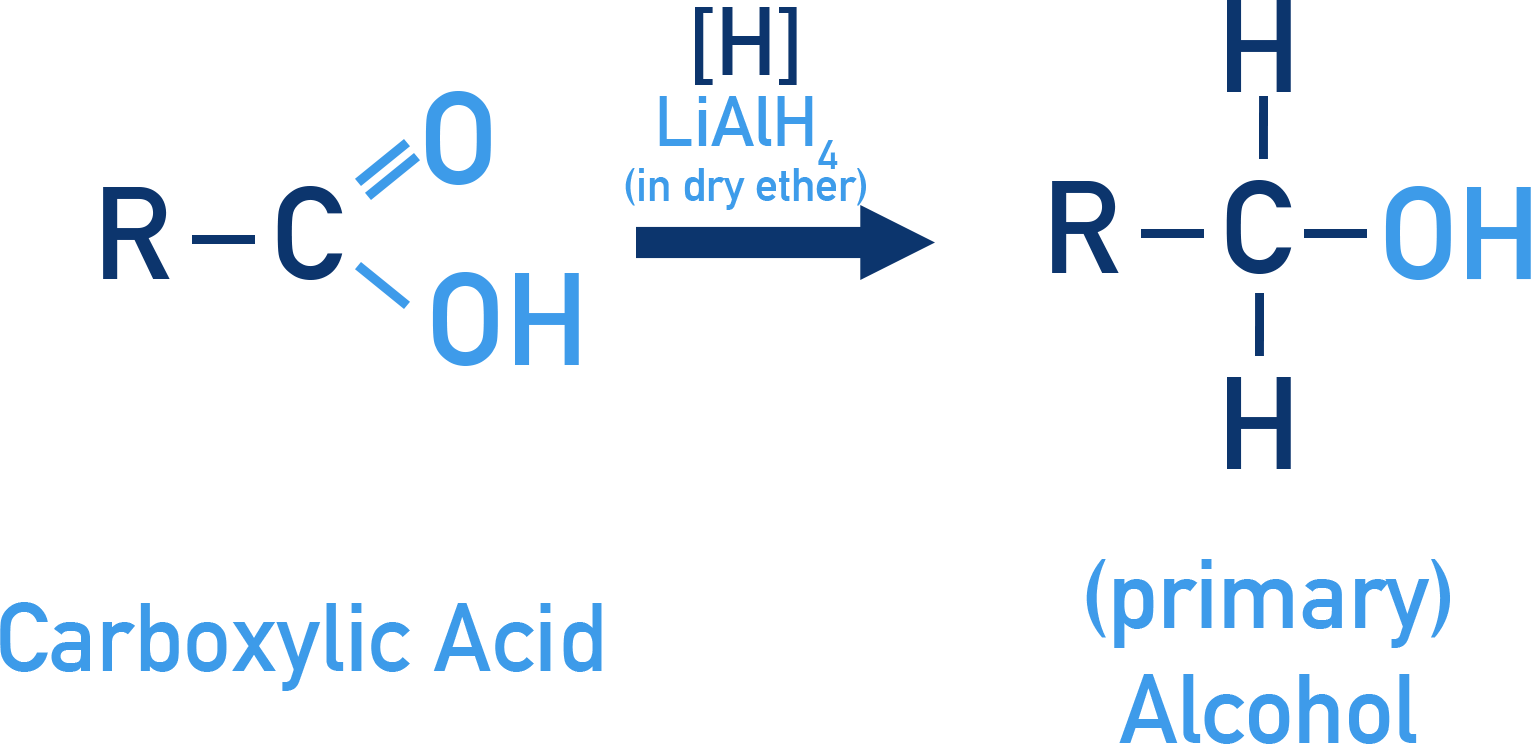
Reagent: LiAlH4 (only)
LiAlH4 is strong enough to reduce acids to primary alcohols, NaBH4 can’t.
LiAlH4 is expensive, so it is typically reserved for the preparation of special chemicals. On a commercial scale, carboxylic acids are first converted to esters (see Section 7.4.4), which are then reduced to alcohols by catalytic hydrogenation – that is, using hydrogen gas in the presence of a catalyst.
Example Ethanoic acid to ethanol
CH3COOH + 4[H] → CH3CH2OH + H2O
Catalytic Hydrogenation
Involves H2 gas and catalysts such as Pd to reduce the C=O bond.
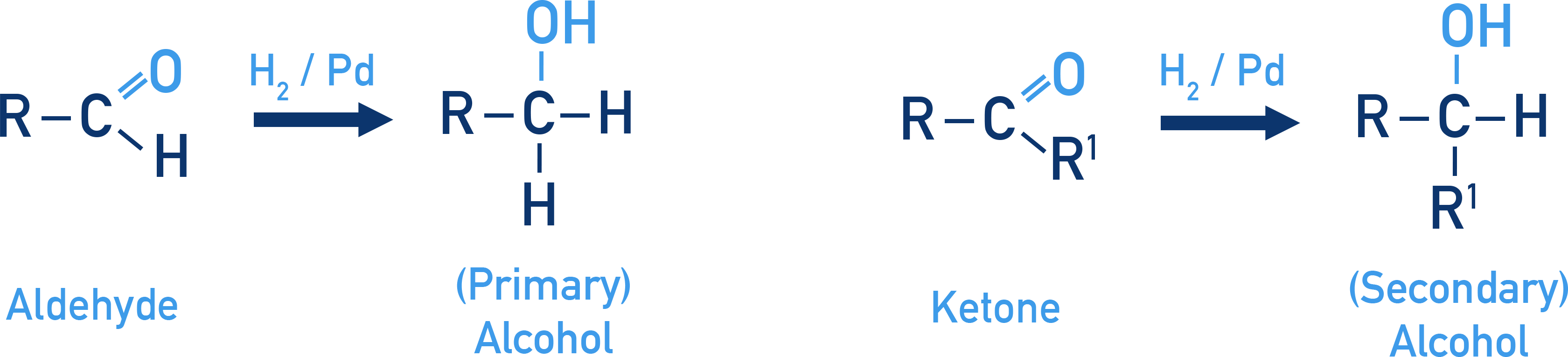
Example Ethanal to ethanol
CH3CHO + H2 → CH3CH2OH
From Grignard Reagents
Grignard reagents react with carbonyl compounds to give alcohols after hydrolysis.
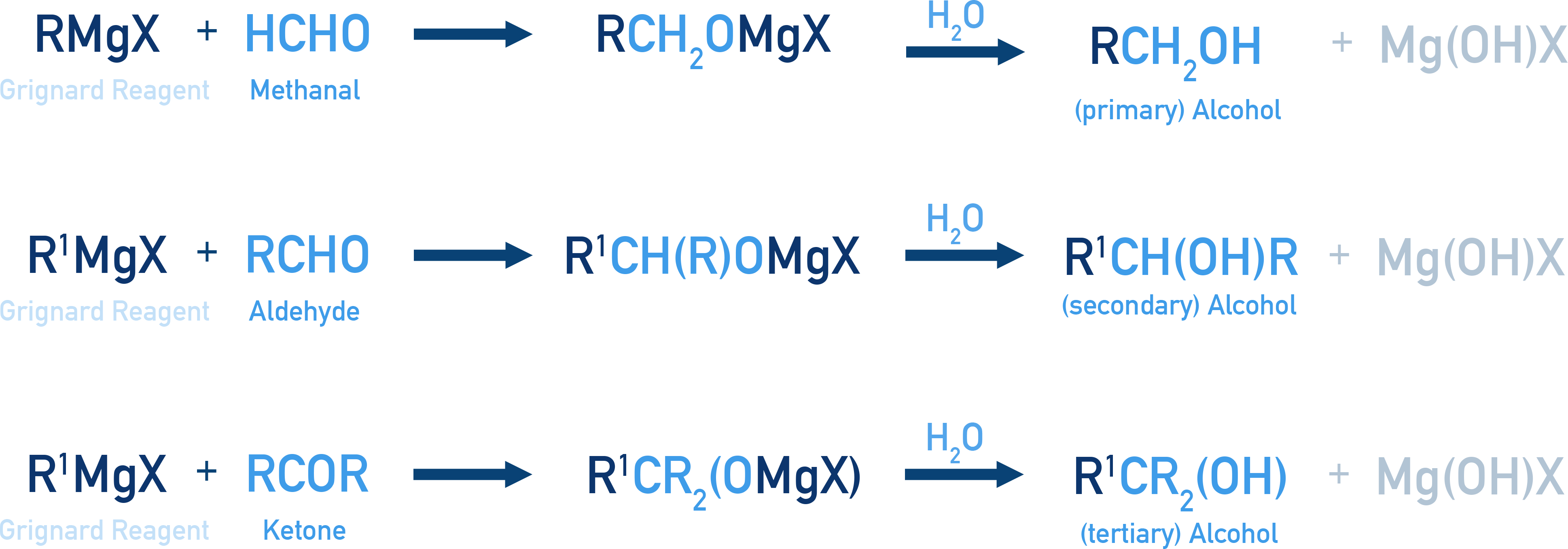
- With Formaldehyde: Forms 1° alcohol
- With Aldehydes: Forms 2° alcohol
- With Ketones: Forms 3° alcohol
Example Propan-2-ol from CH3MgBr
CH3MgBr + CH3CHO → CH3CH(OH)CH3
Preparation of Phenols
Phenols are typically obtained from aromatic compounds through substitution or hydrolysis reactions.
Phenols From Haloarenes
Nucleophilic substitution of halogen by –OH under harsh conditions.
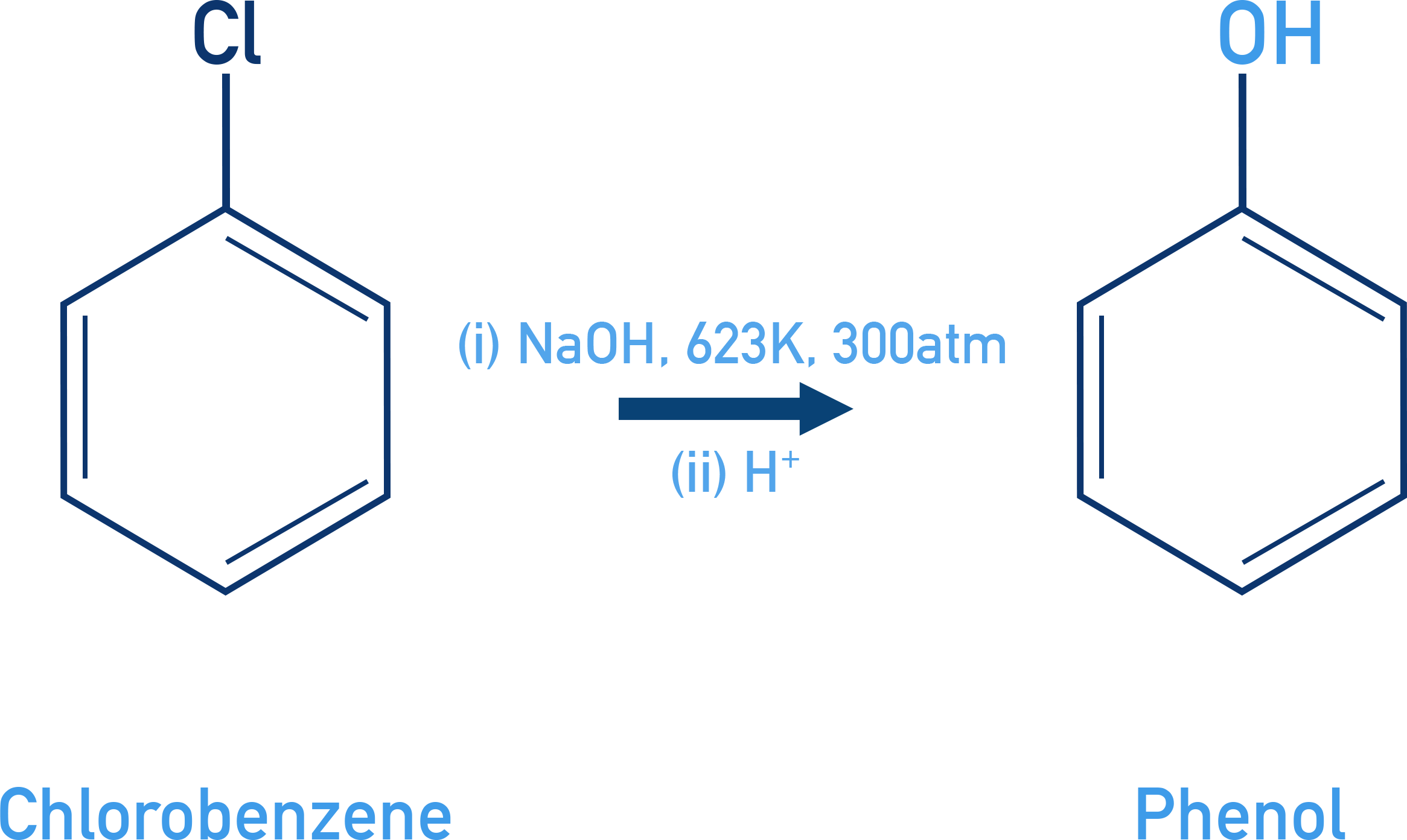
Reagent: NaOH, 623 K, 300 atm
More efficient with electron-withdrawing groups (e.g., NO2) at ortho/para positions.
Example Chlorobenzene to phenol
C6H5Cl → C6H5OH
Phenols From Benzenesulphonic Acid
Alkaline fusion followed by acidification gives phenol.
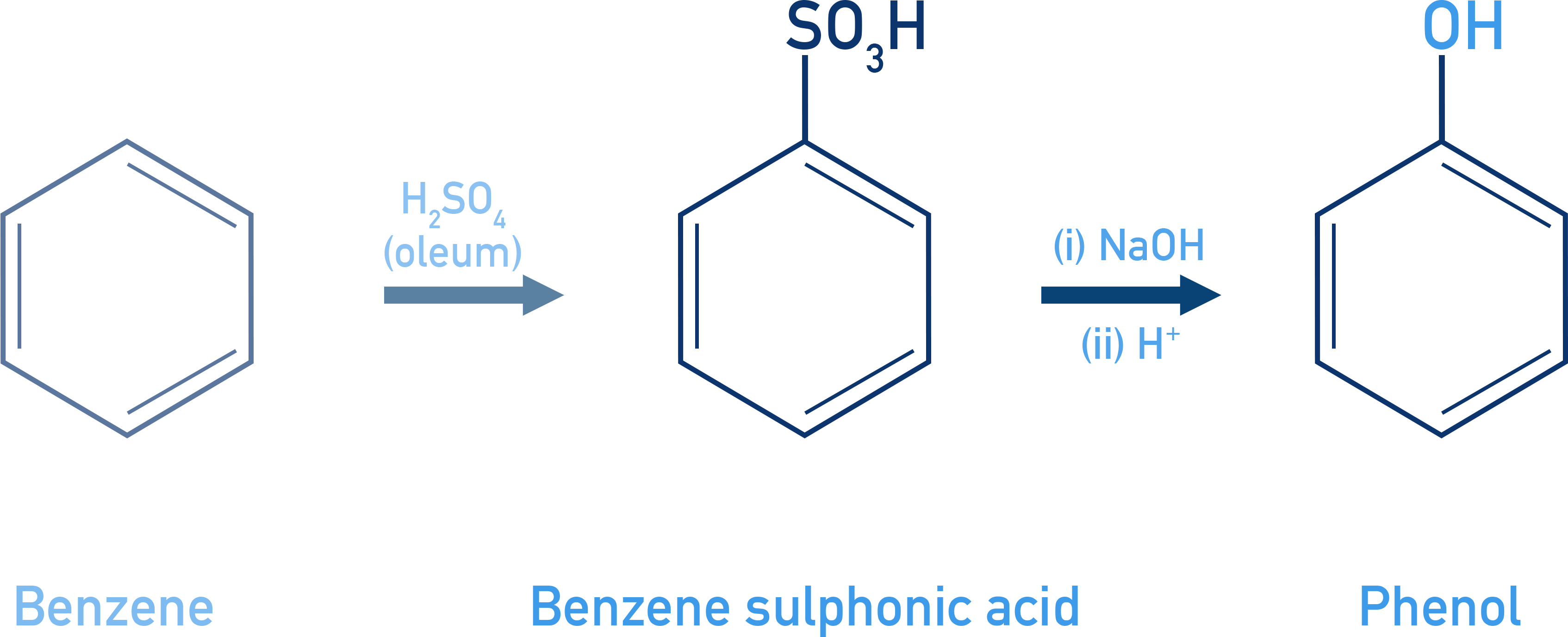
- Step 1: C6H5SO3H + NaOH → C6H5ONa
- Step 2: C6H5ONa + HCl → C6H5OH + NaCl
Phenols From Diazonium Salts
Thermal decomposition of diazonium salt yields phenol.
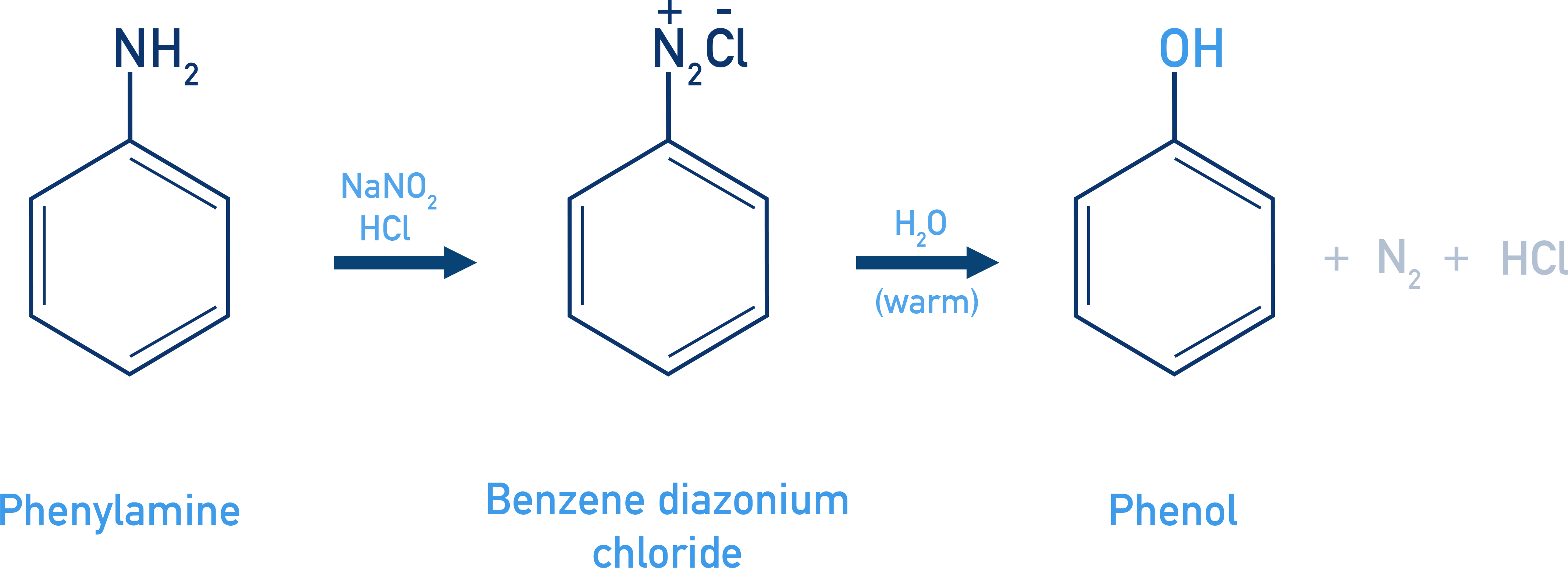
Reagent: H2O or Cu2O/HCl
Example Benzenediazonium chloride
C6H5N2+Cl− + H2O → C6H5OH + N2 + HCl
Phenols From Cumene
Industrial method involving oxidation of cumene and hydrolysis.
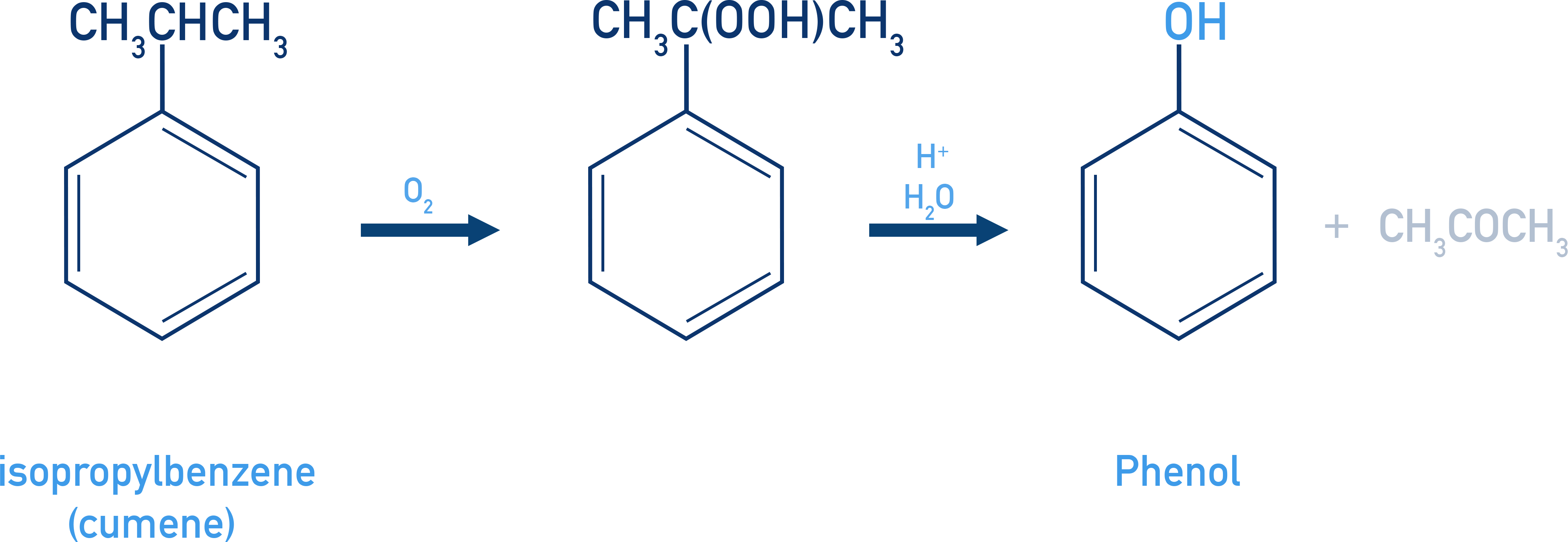
- Cumene + O2 → Cumene hydroperoxide
- Hydrolysis with a dilute acid → Phenol + Acetone
Physical Properties
Boiling Points
Due to hydrogen bonding, alcohols and phenols have higher boiling points than hydrocarbons of similar size.
- Boiling points increase with:
- Increase in number of carbon atoms (due to stronger van der Waals forces).
- Boiling points decrease with:
- Increase in branching of the carbon chain (branching reduces surface area and van der Waals interactions between molecules).
Intermolecular Hydrogen Bonding:
Alcohols and phenols have an –OH group capable of forming strong hydrogen bonds with each other.
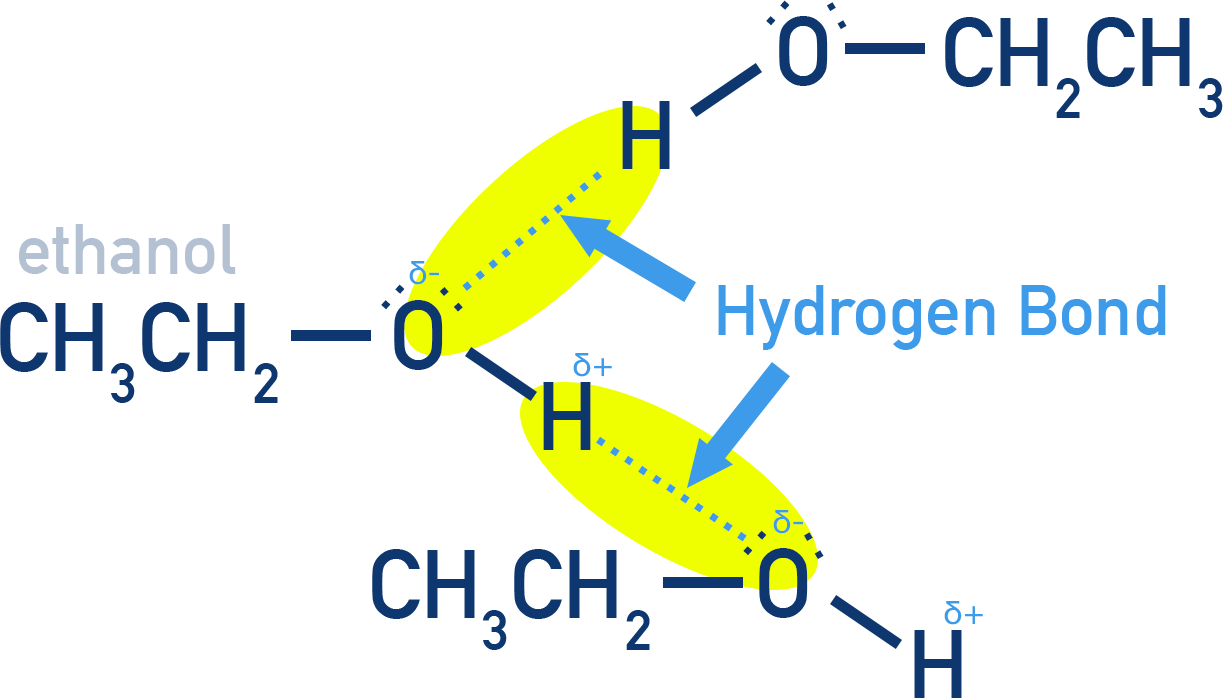
These hydrogen bonds raise their boiling points significantly.
Solubility
Hydrogen bonding makes shorter chain alcohols and phenols water-soluble.
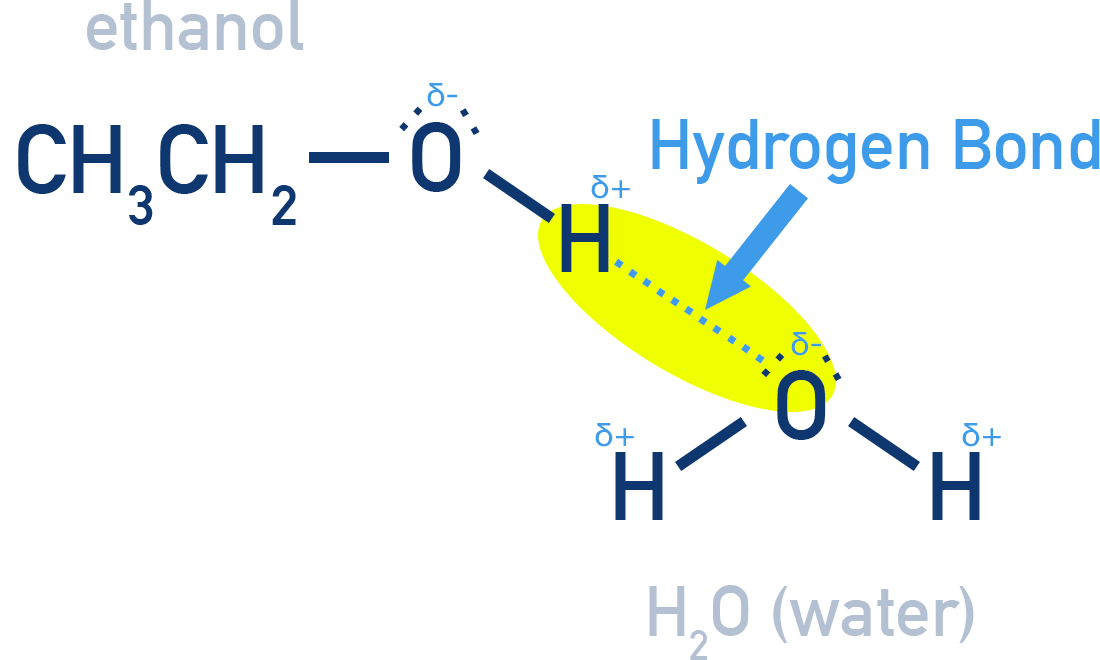
Solubility decreases with increasing alkyl group size. Polyhydric alcohols (those with more than one OH group) are more soluble than monohydric ones.
Chemical Reactions
Alcohols are versatile and can behave as Nucleophiles (electron pair donors) and Electrophiles (electron pair acceptors after protonation)
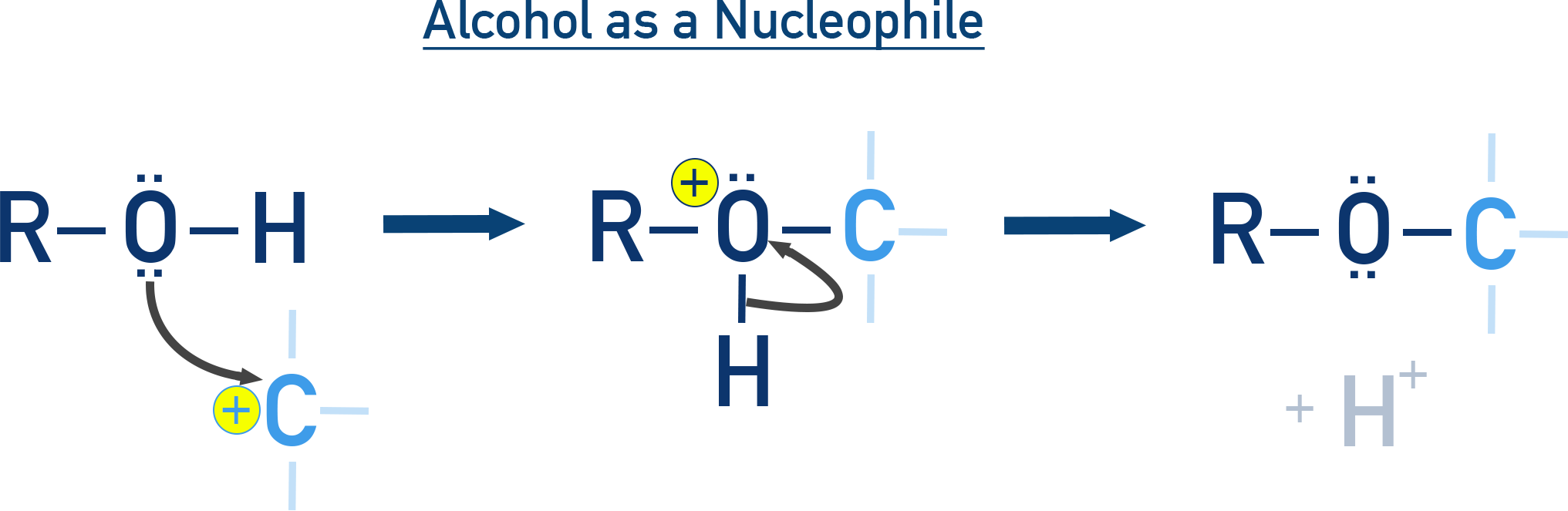
Alcohols as Nucleophiles:
The O–H bond is broken.
The lone pair on oxygen attacks an electrophile (e.g. a carbon with a leaving group).
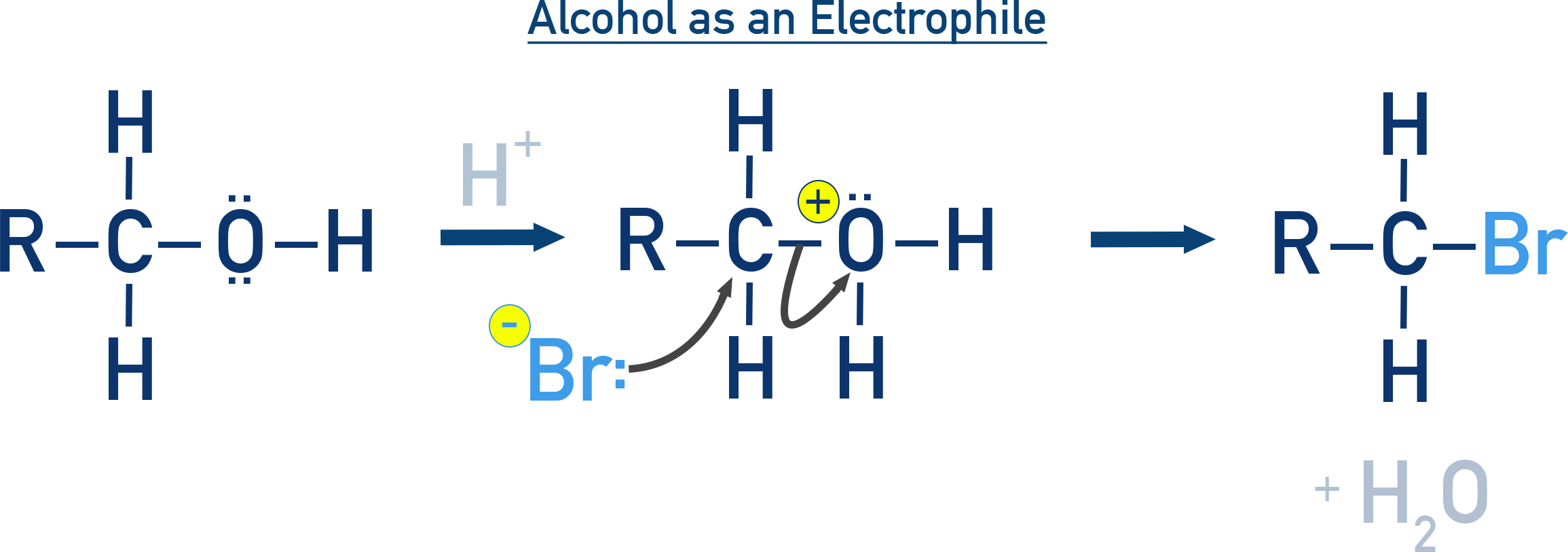
Protonated Alcohols as Electrophiles:
The C–O bond is broken after protonation of the alcohol.
The alcohol becomes –OH2+, a good leaving group.
Reactions Involving Cleavage of O–H Bond
Reaction with Metals:
Alcohols and phenols react with Na, K, Al to form alkoxides/phenoxides + H2.
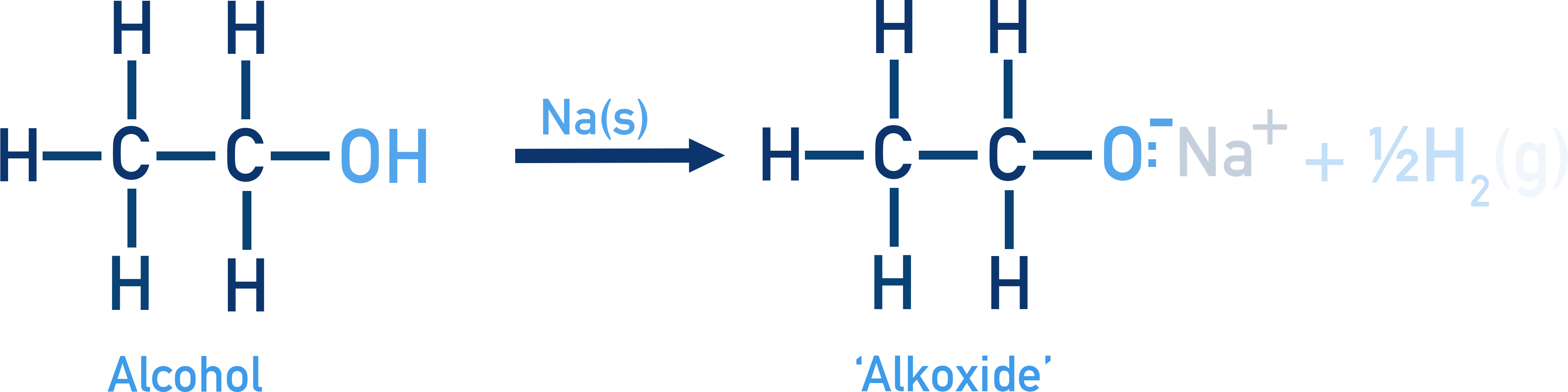
2R–OH + 2Na → 2R–ONa + H2
Phenol + Na → Sodium phenoxide + H2
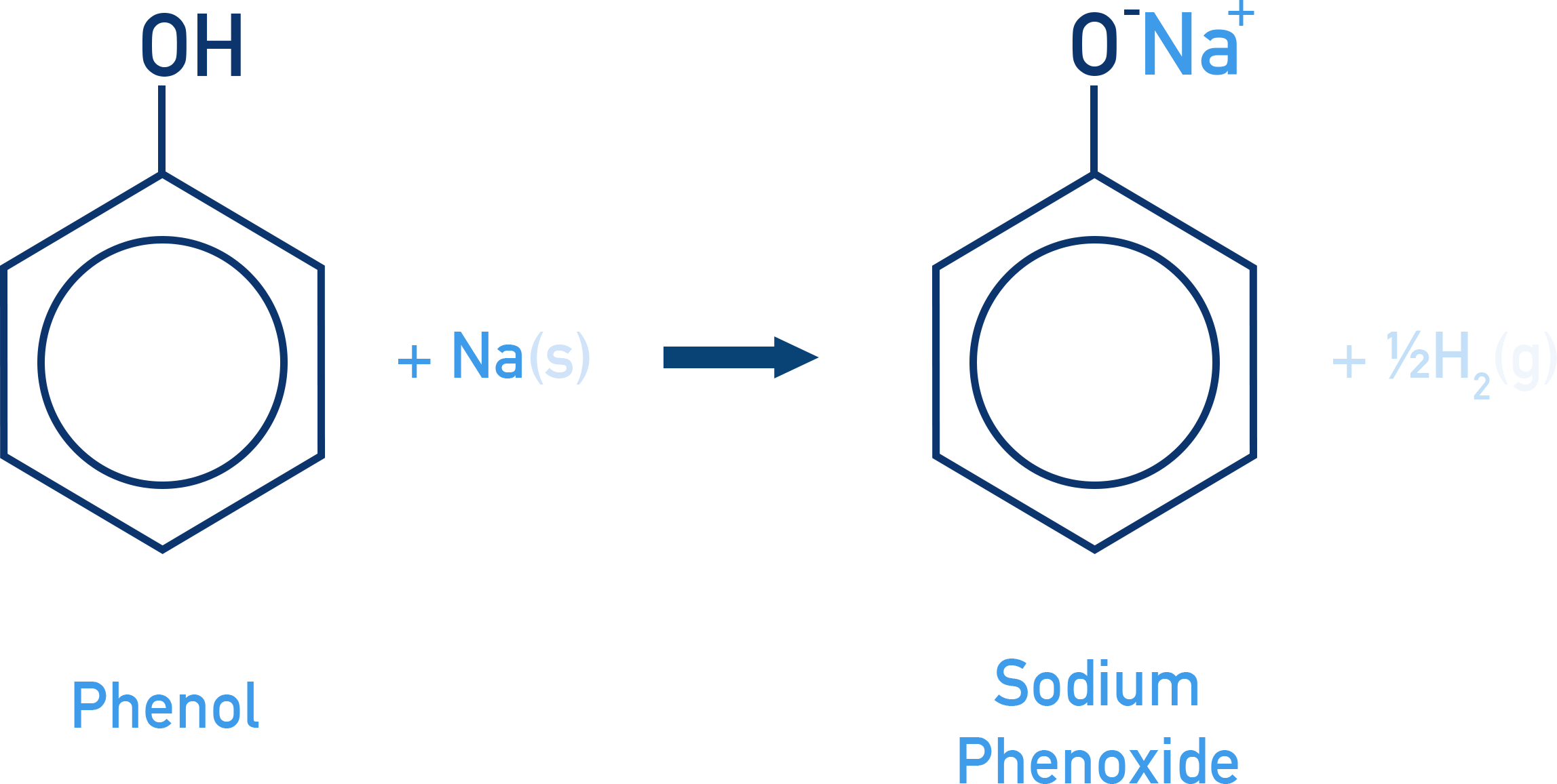
Reaction with Aqueous NaOH:
Phenols react with NaOH forming sodium phenoxide.
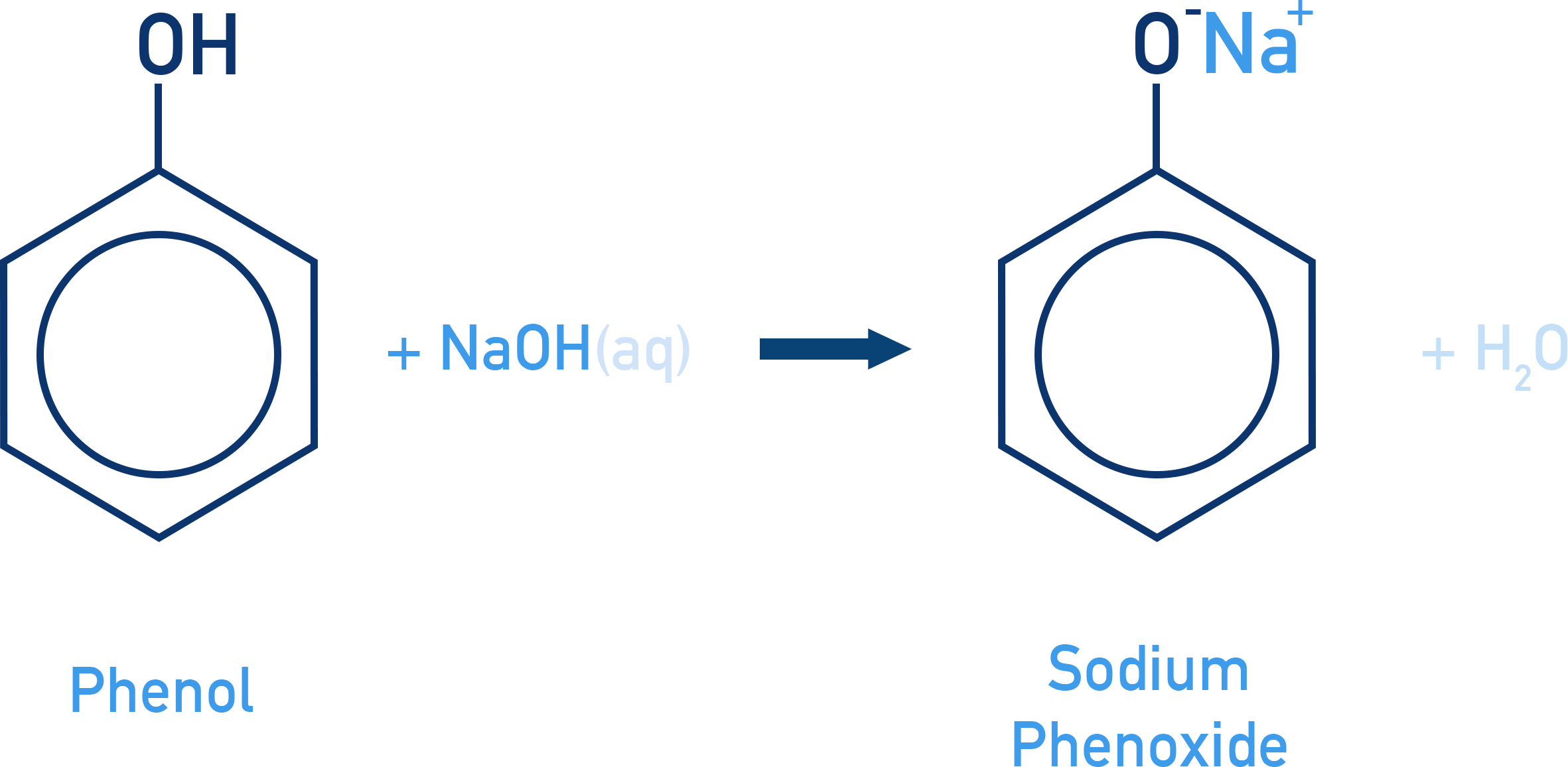
Alcohols do not react (less acidic).
Nature of Acidity:
Alcohols & phenols are Brønsted acids (proton donors).
Acidity order (due to electron-donating groups reducing O–H polarity):
Primary > Secondary ≫ Tertiary
Comparison with Water:
Alcohols are weaker acids than water. This is because the alkoxide ion is a stronger base than OH−. This can be seen between the reaction of an alkoxide ion and water, forming an alcohol (the conjugate acid of the alkoxide ion) and OH− ions.

Phenol Acidity:
Due to the sp2 carbon of benzene ring (electron withdrawing). Resonance stabilizes phenoxide ion → increases acidity.
Ionisation Comparison:
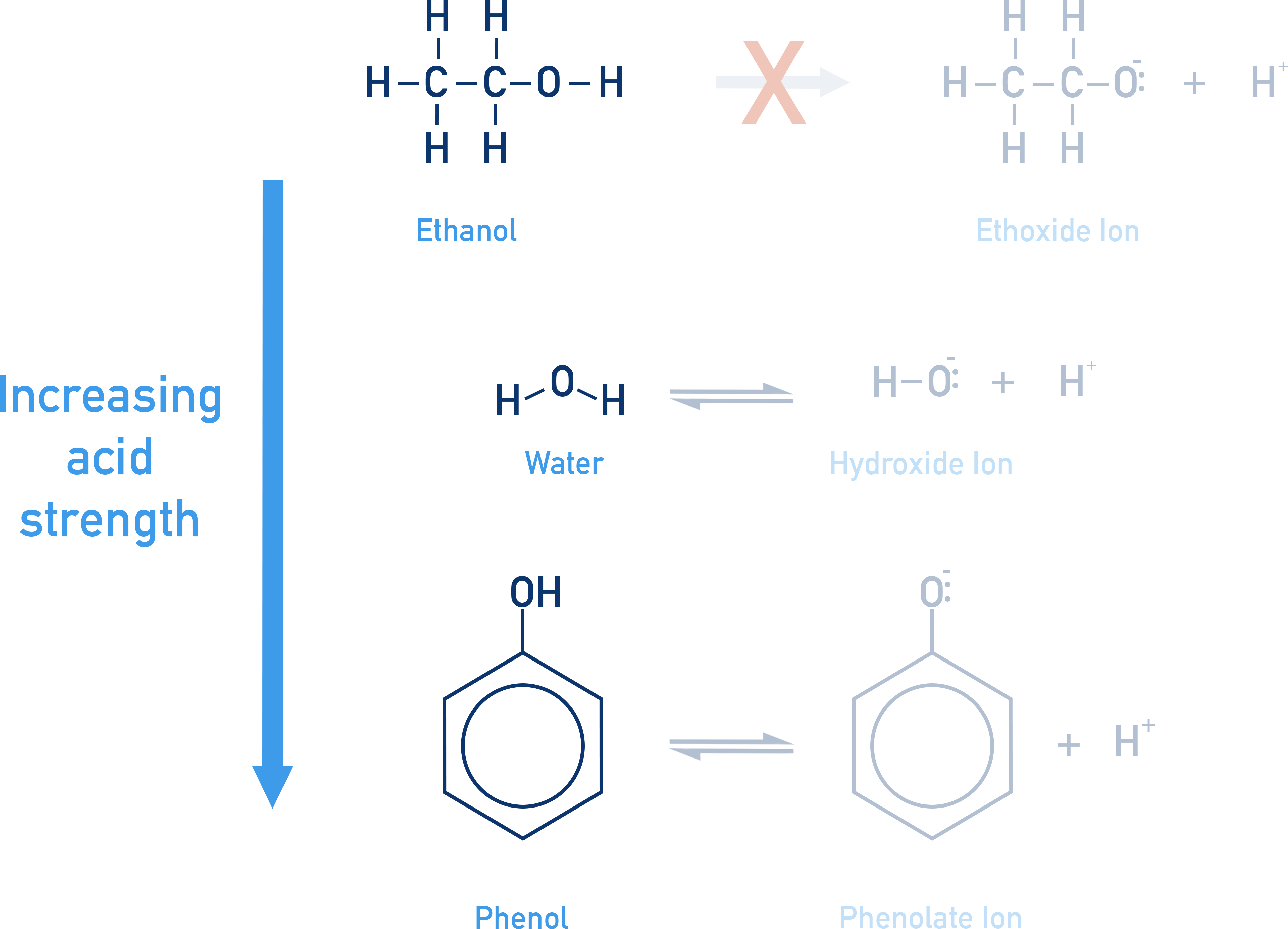
- Alcohol: R–OH ⇌ R–O− + H+
- Phenol: Ar–OH ⇌ Ar–O− + H+
- Phenoxide ion: Resonance stabilized.
- Alkoxide ion: Localized charge only.
Substituent Effects:
Electron-withdrawing groups (e.g., –NO2) at ortho/para increase acidity. Electron-donating groups (e.g., –CH3) decrease acidity.
Reactions Involving Cleavage of C–O Bond (Alcohols only)
Reaction with HX (Lucas Test):
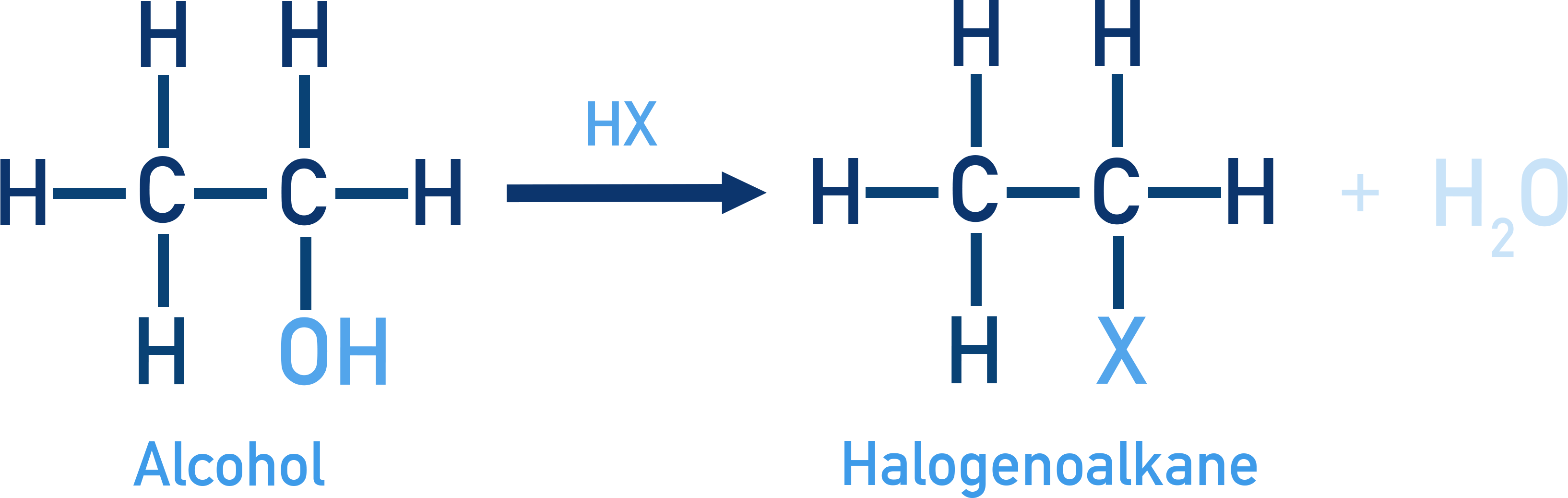
ROH + HX → R–X + H2O
Lucas reagent: conc. HCl + ZnCl2.
- Tertiary alcohols → immediate cloudiness
- Secondary → slower change to cloudiness
- Primary → colourless at room temperature
Reaction with PX3:
ROH + PX3 → R–X (see class 12, section 6.4)
Dehydration to Alkenes:
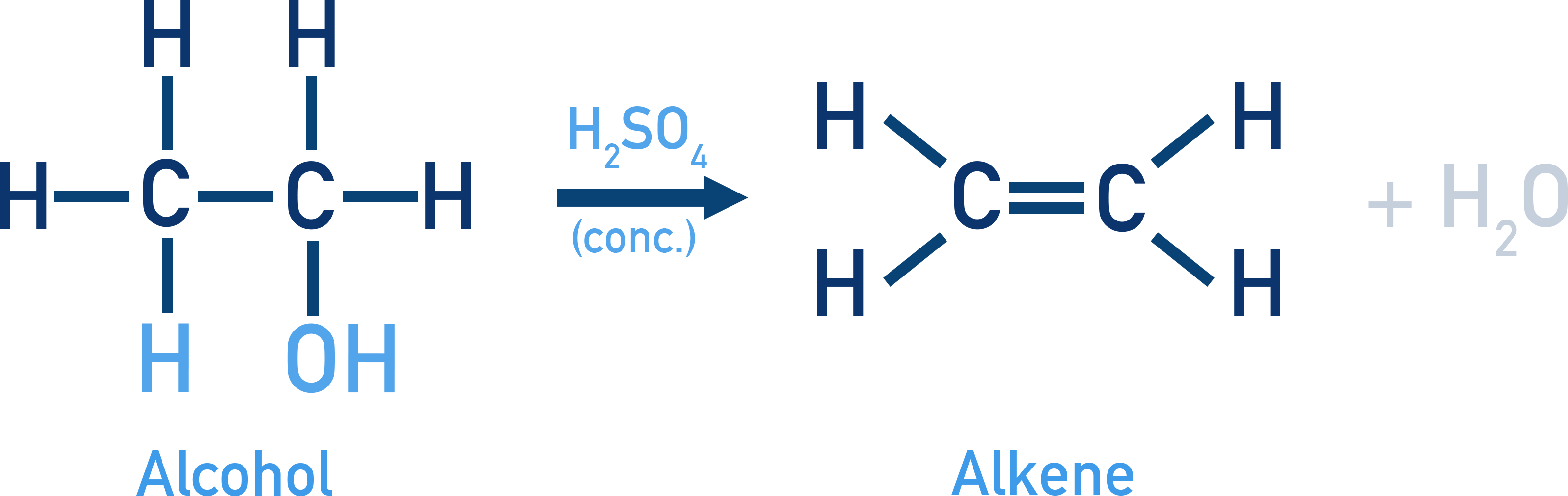
Reagent: Conc. H2SO4 / H3PO4 / Al2O3 (heat)
Order of ease of dehydration: Tertiary > Secondary > Primary (conditions required to dehydrate get milder)
Mechanism (Ethanol example):
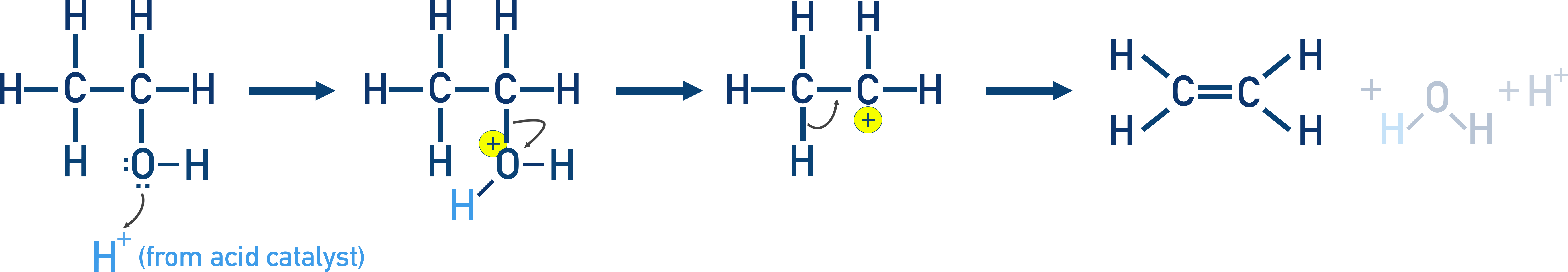
- Protonation of OH
- Formation of carbocation (slow)
- Elimination of H+ → alkene formed
Oxidation of Alcohols
Oxidation of alcohols involves the removal of hydrogen (dehydrogenation) or the addition of oxygen. It typically affects the O–H and C–H bonds on the carbon bearing the –OH group.
Primary alcohols (1°)
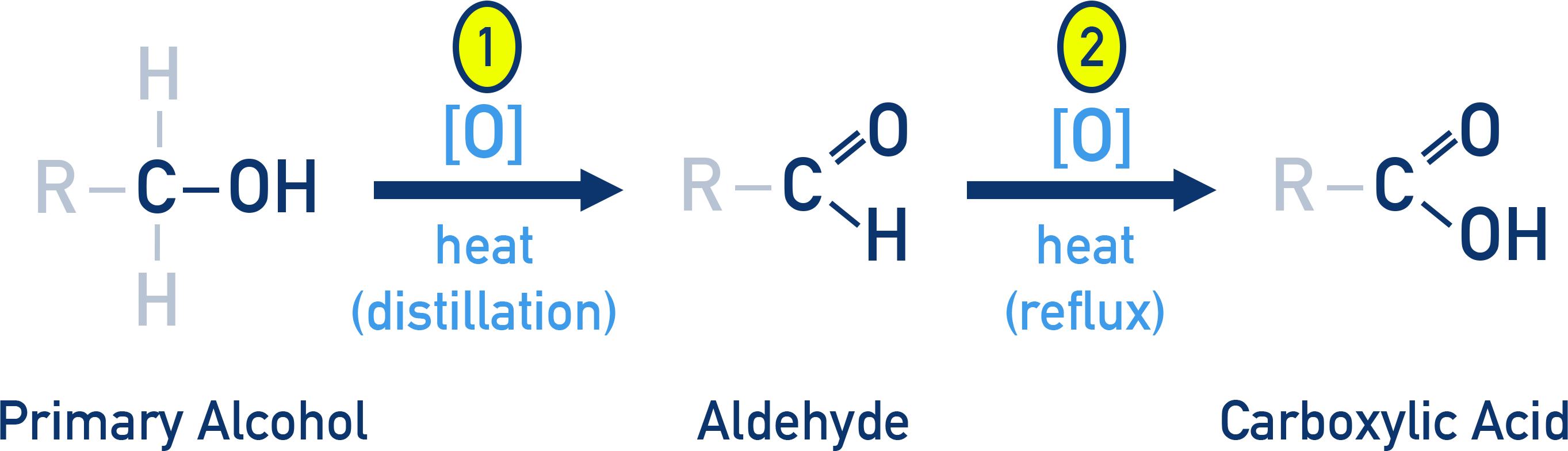
- Undergo two-step oxidation:
- → Aldehyde
- → Carboxylic acid (with strong oxidising agents)
- Reagents:
- Strong oxidants ([O]) like acidified KMnO4 or K2Cr2O7 oxidise primary alcohols completely to carboxylic acids.
- Chromium trioxide (CrO3) in anhydrous medium can oxidise to aldehydes.
- PCC (Pyridinium chlorochromate) is a mild, selective reagent that stops oxidation at the aldehyde stage.
Secondary alcohols (2°)
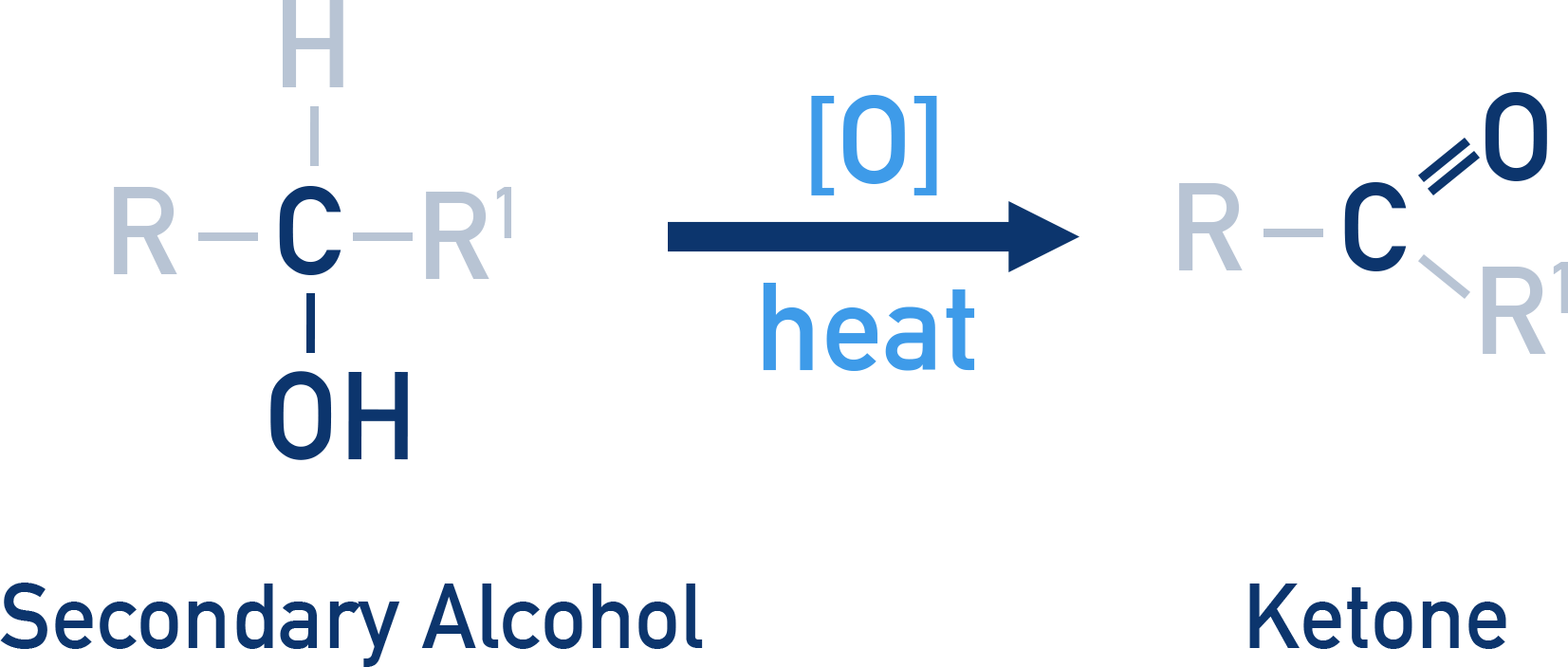
- Oxidised to ketones.
- Cannot be further oxidised to carboxylic acids (no hydrogen on the carbon bearing –OH).
- CrO3 is commonly used for this oxidation.
Tertiary alcohols (3°)
Do not oxidise easily because they lack a hydrogen atom on the carbon with the –OH group.
Under drastic conditions (e.g., strong oxidisers like KMnO4 at high temperatures), they undergo cleavage of C–C bonds, forming a mixture of carboxylic acids with smaller carbon chains.
Dehydrogenation (Catalytic oxidation at 573 K with Cu)
Dehydrogenation is used in laboratory preparation of aldehydes and ketones without over-oxidising the product.
Alcohol vapours are heated over copper:
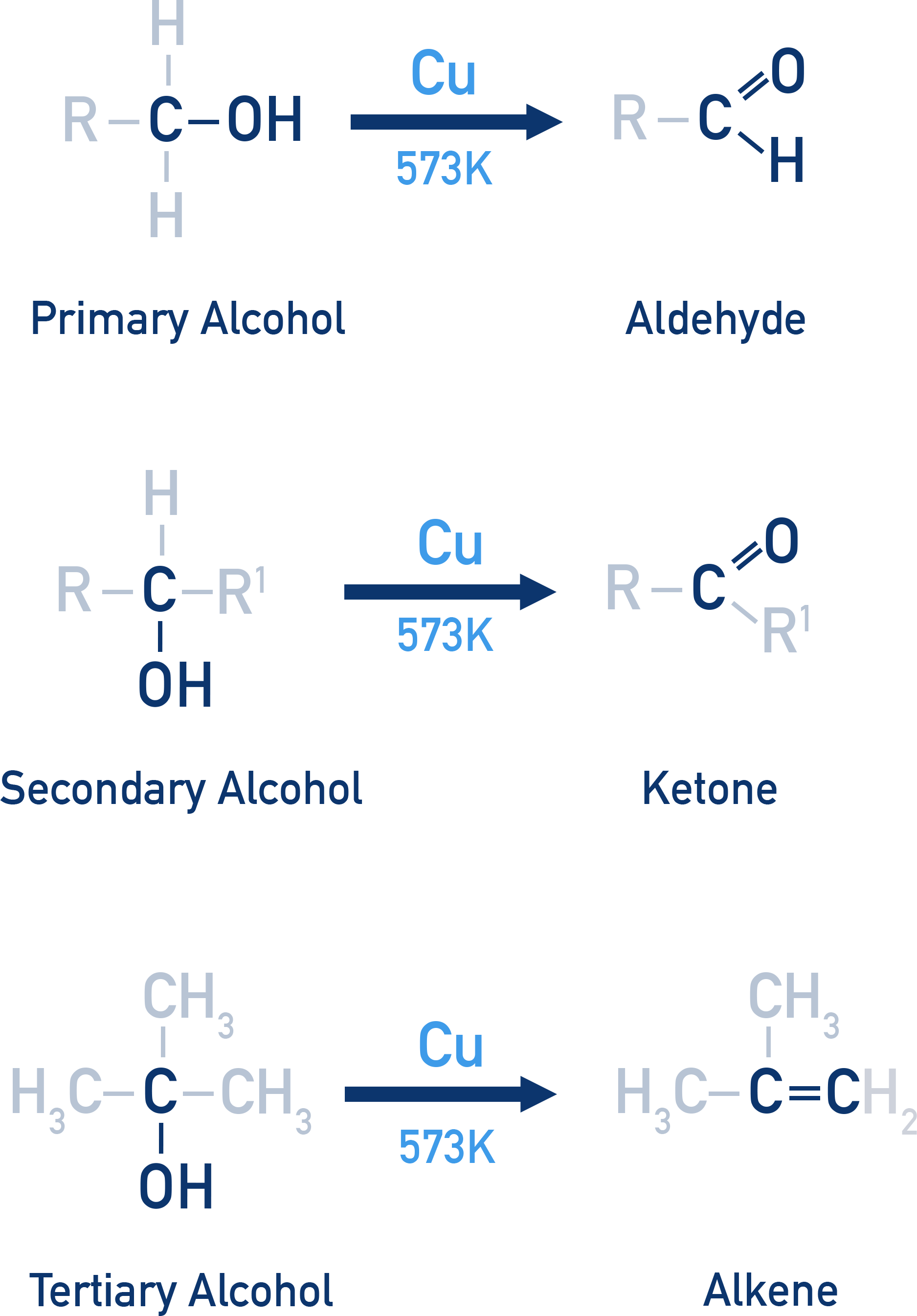
- Primary alcohols → Aldehydes
- Secondary alcohols → Ketones
- Tertiary alcohols → Alkenes (via dehydration, not oxidation)
Reactions of Phenols
Phenol is more reactive than benzene due to the lone pair on the oxygen delocalising into the π-system.
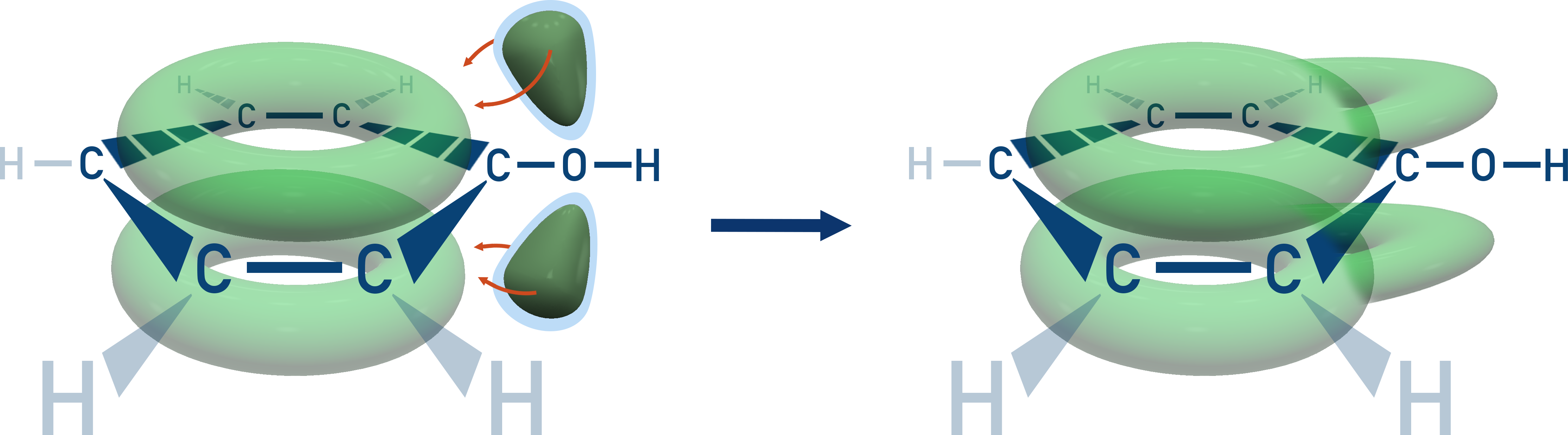
This increases electron density, activating the ring towards electrophilic substitution.
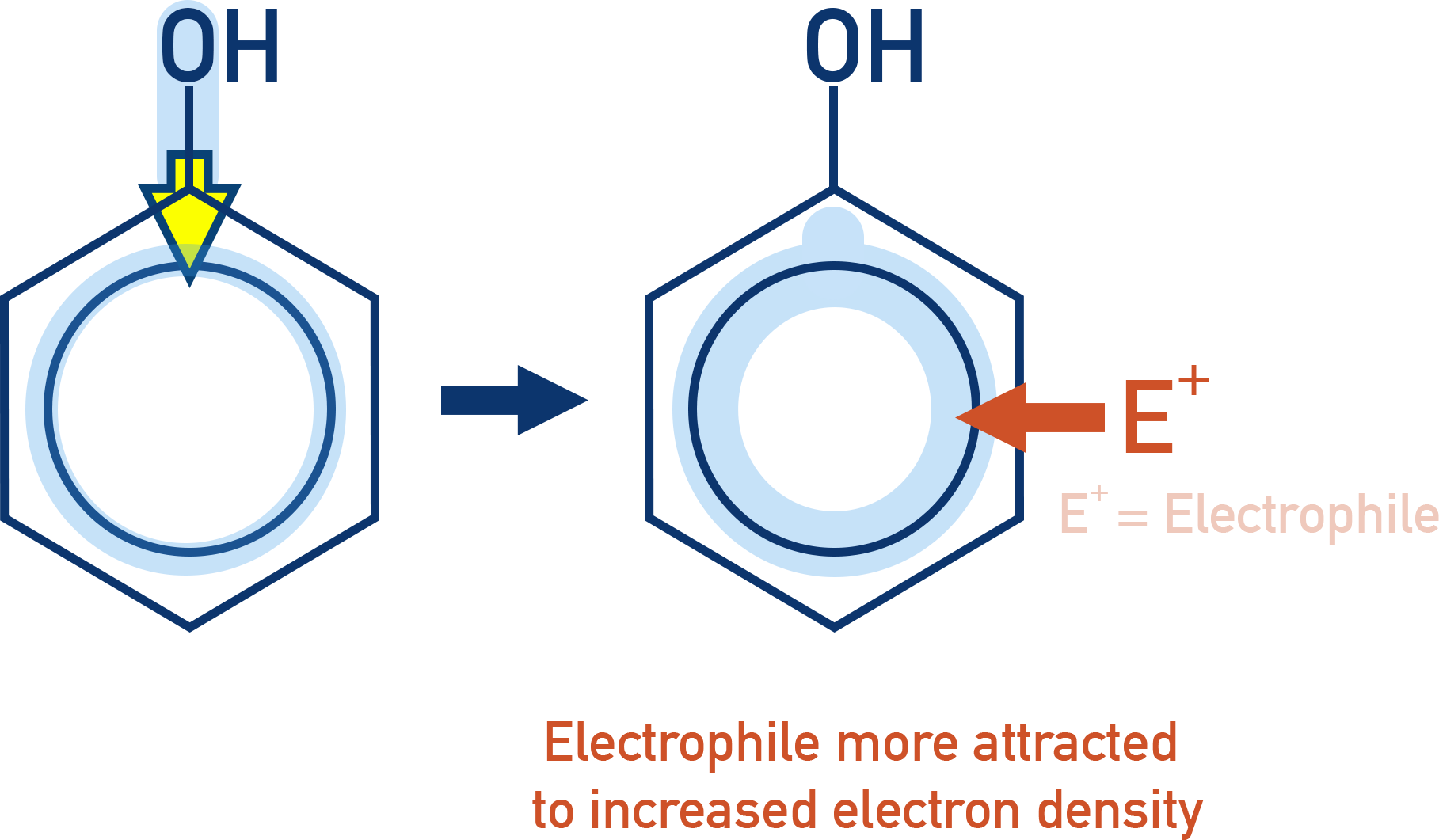
- Therefore, phenol reacts under milder conditions than benzene (e.g. nitration without a catalyst).
- –OH activates ring (ortho/para directing)
Directing Effects of the –OH Group
The hydroxyl group activates the benzene ring and directs substitution to the ortho (2-), para (4-), and 6-positions.
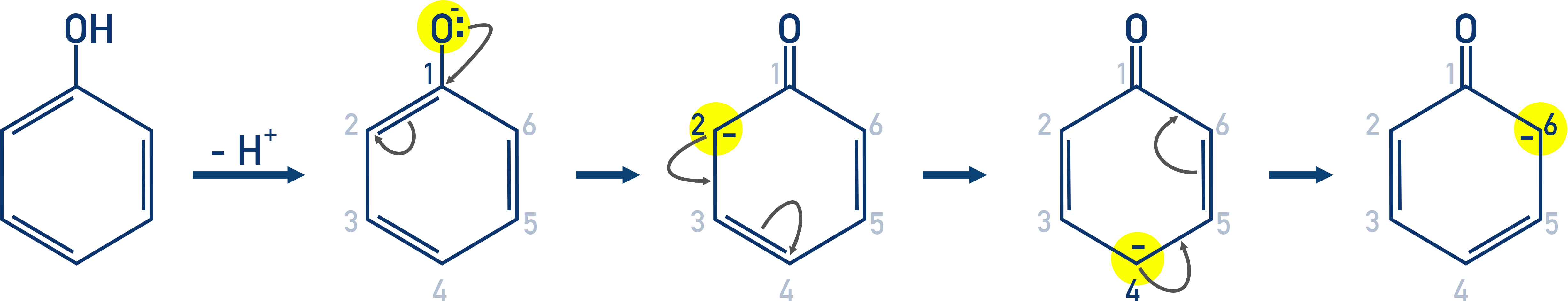
This explains the pattern of substitution.
Nitration:
With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols.
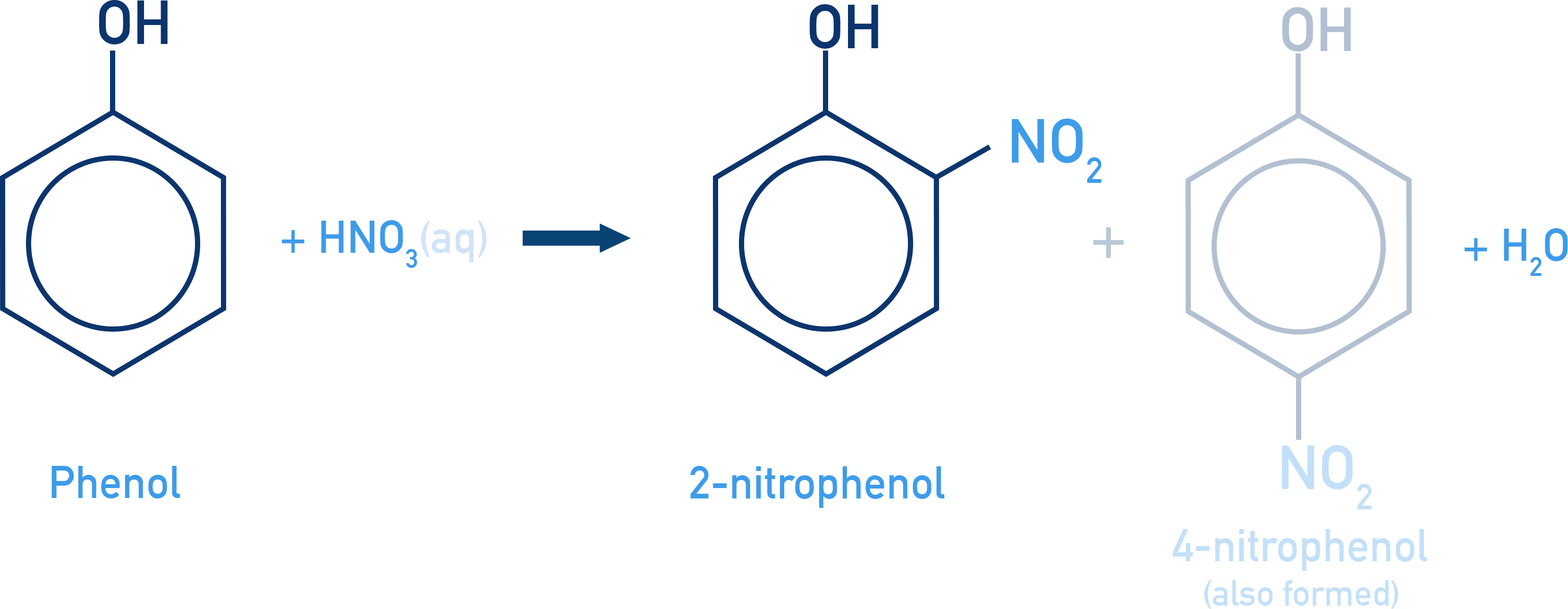
The positional isomers can be separated by steam distillation because 2-nitrophenol (o-nitrophenol) is more volatile than 4-nitrophenol (p-nitrophenol).
This is due to intramolecular hydrogen bonding in 2-nitrophenol, which does not hinder volatility, while intermolecular hydrogen bonding in 4-nitrophenol leads to stronger molecular interactions, reducing its volatility.
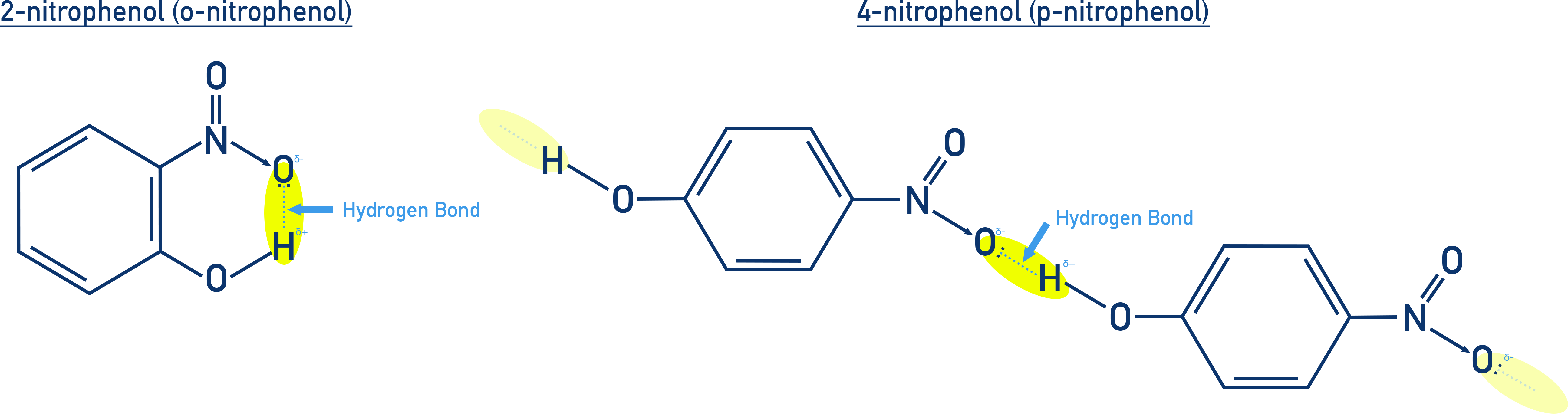
- o-Nitrophenol: Intramolecular H-bond = more volatile
- p-Nitrophenol: Intermolecular H-bond = less volatile
When concentrated HNO3 is used, multiple substitutions occur and Picric acid (2,4,6-trinitrophenol) is formed.
Halogenation:
Phenol reacts with bromine to give different products depending on the reaction conditions used.
In CS2 and low temperatures, mono bromination occurs (only one Br gets substituted into the ring).

In bromine water, multiple bromination occurs, forming 2,4,6-tribromophenol (a white ppt)
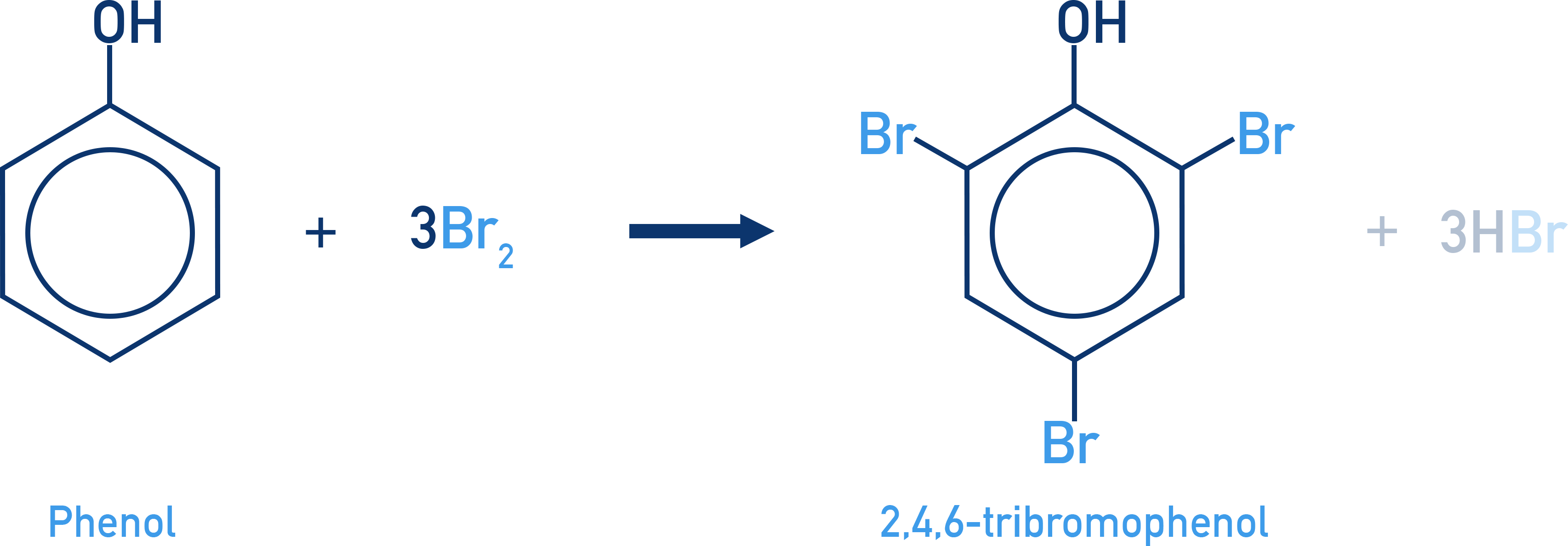
Note that, unlike benzene, phenol does not require a Lewis acid catalyst for bromination. The electron-donating –OH group activates the aromatic ring sufficiently to polarise Br2 molecules and generate an electrophile, enabling the reaction without the need for a catalyst.
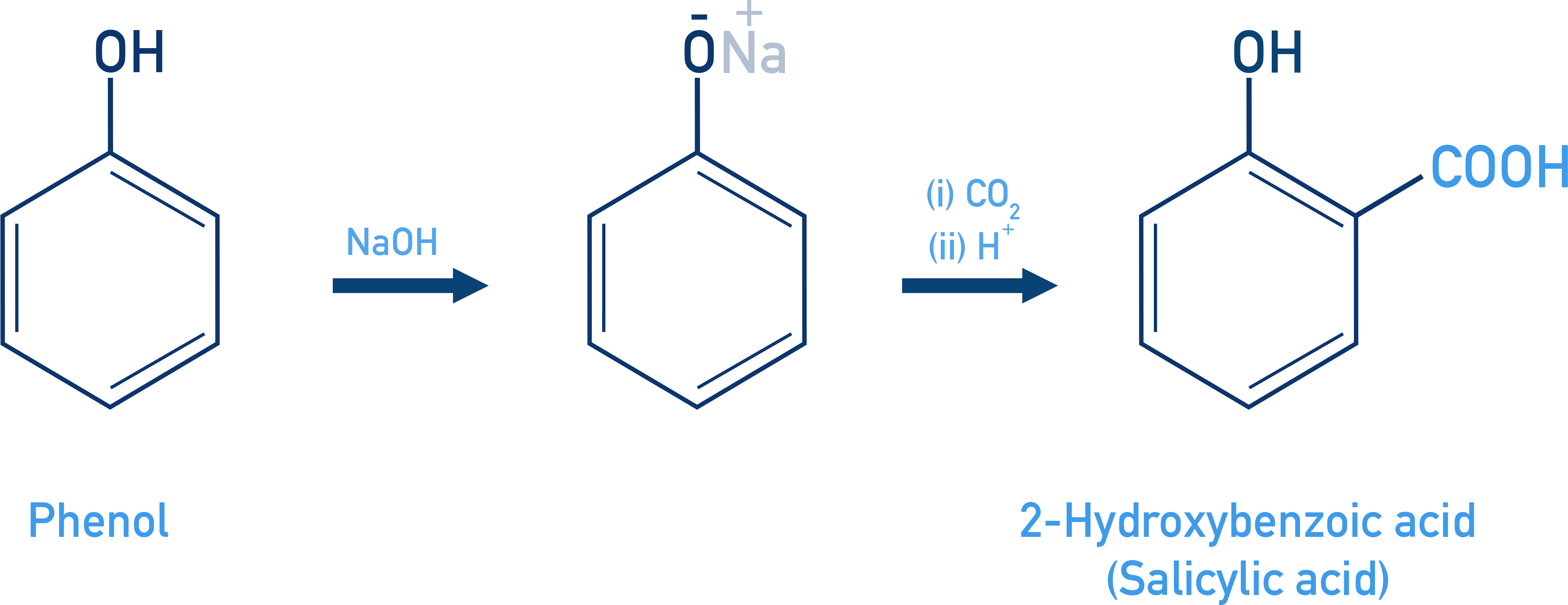
Kolbe’s Reaction:
When phenol is treated with sodium hydroxide, it forms the phenoxide ion, which is even more reactive than phenol in electrophilic aromatic substitution. This increased reactivity allows it to react with carbon dioxide – a relatively weak electrophile – resulting in the formation of ortho-hydroxybenzoic acid as the major product.
Reimer-Tiemann Reaction:
When phenol is treated with chloroform (CHCl3) in the presence of sodium hydroxide, a -CHO group is introduced at the 2 (ortho) position of the aromatic ring. This reaction is called the Reimer–Tiemann reaction.
The reaction proceeds via the formation of a substituted benzal chloride intermediate, which is then hydrolysed under alkaline conditions to yield salicylaldehyde (2-hydroxybenzaldehyde) as the final product.
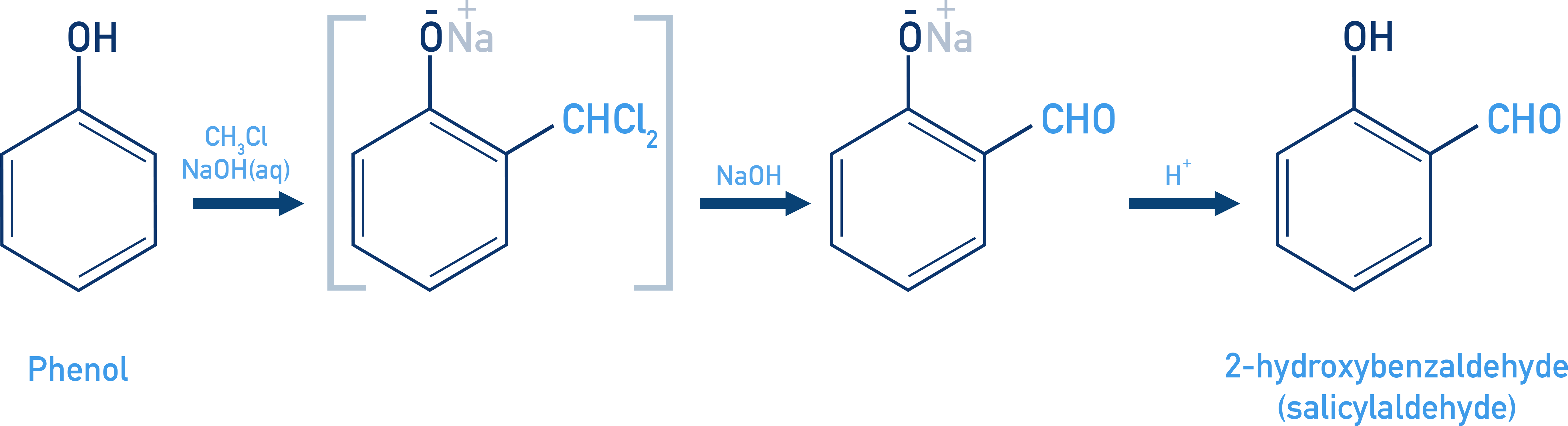
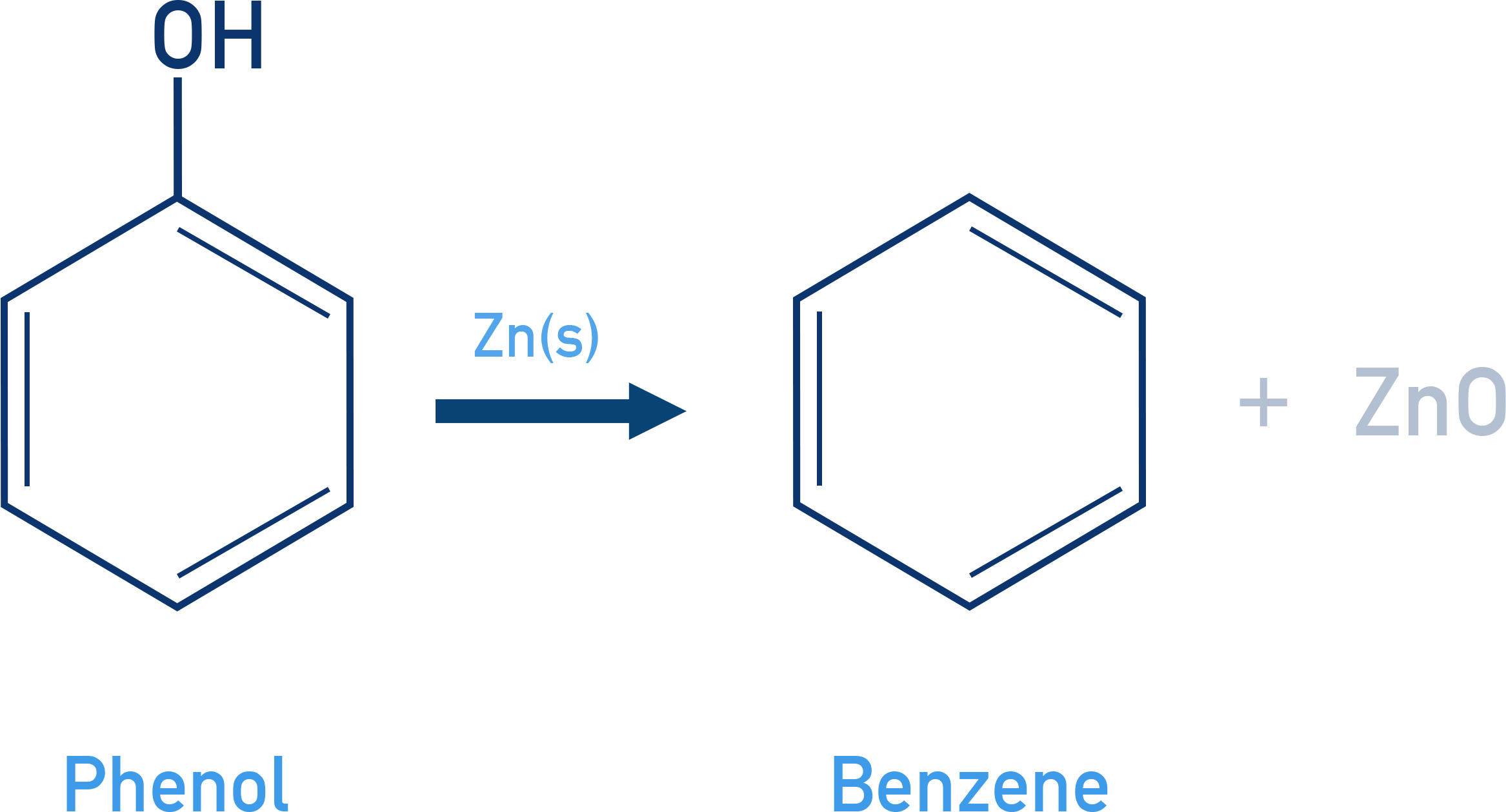
Reaction with Zn Dust:
When phenol is reacted with zinc dust, benzene is formed.
Oxidation:
When phenol is oxidised with chromic acid, it forms a conjugated diketone called benzoquinone.
Phenols also undergo slow oxidation when exposed to air, producing dark-coloured mixtures that contain quinone derivatives.
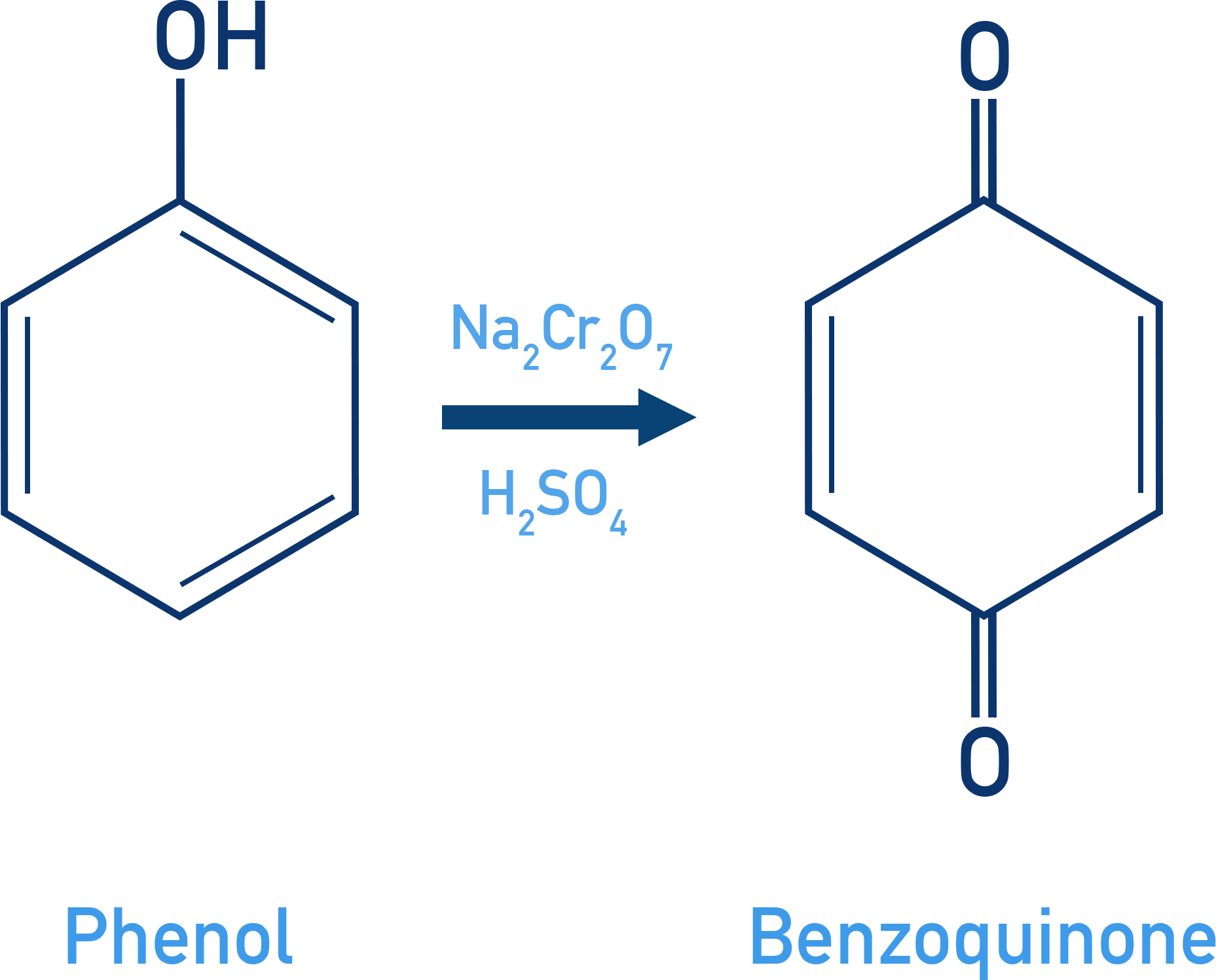
Phenol + Na2Cr2O7/H2SO4 → Benzoquinone (diketone)
Air oxidation → dark-colored quinones
Summary
- Alcohols form from alkenes, carbonyl reductions, and Grignard additions while phenols form from haloarenes, sulphonates, diazonium salts, and cumene.
- Hydrogen bonding raises boiling points and small chains are more soluble in water.
- Alcohols show O–H acidity and undergo C–O substitutions and dehydration; oxidation depends on degree of the alcohol.
- Phenol is activated to ortho and para substitution and undergoes nitration, bromination, Kolbe’s and Reimer–Tiemann reactions.
