Electrons, energy levels and atomic orbitals
Quick Notes
- Electrons exist in energy levels (shells), which contain sub-shells (s, p, d) made up of orbitals.
- Each orbital can hold 2 electrons with opposite spins.
- s orbitals are spherical; p orbitals are dumbbell shaped.
- Sub-shells:
- s has 1 orbital (2 electrons)
- p has 3 orbitals (6 electrons)
- d has 5 orbitals (10 electrons)
- Energy levels are labelled by the principal quantum number (n): n = 1, 2, 3...
- Electrons fill sub-shells in order of increasing energy: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p
- Electronic configurations can be shown fully (e.g. 1s2 2s2 …) or using shorthand (e.g. [Ar] 3d6 4s2).
- Electrons-in-boxes notation shows individual orbitals and spin pairing.
- Free radicals are species with one unpaired electron.
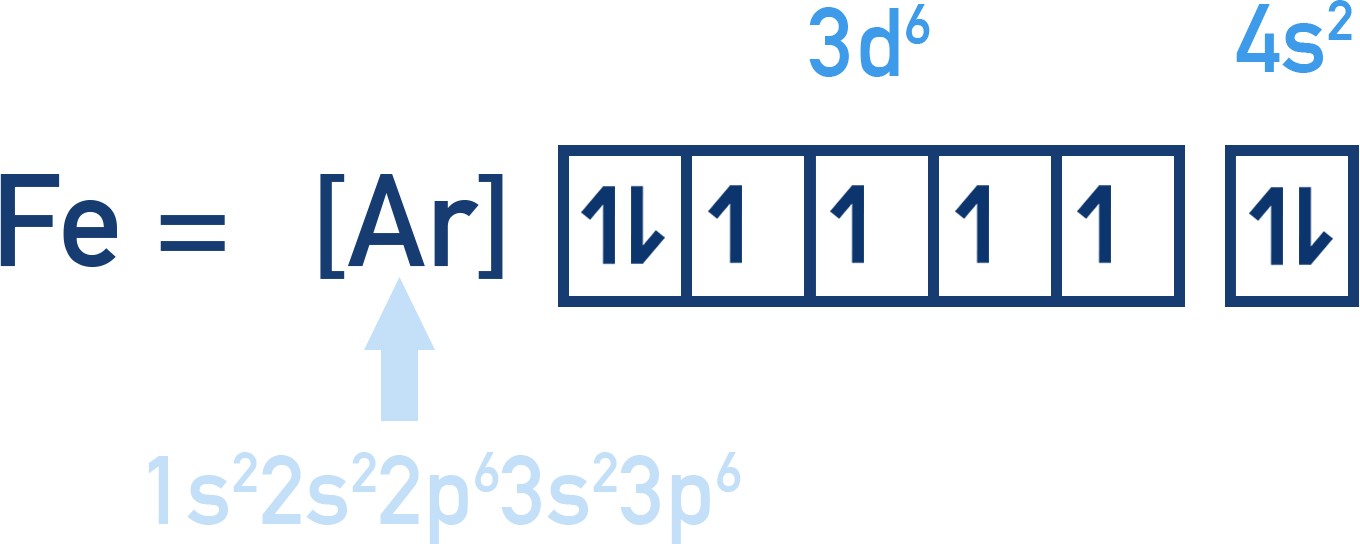
Full Notes
Electron configurations and orbital shapes have been outlined in more detail here and here.
This page is just what you need to know for CIE A-level Chemistry :)
Shells, Sub-shells, Orbitals and the Principal Quantum Number (n)
Electrons in an atom are arranged in energy levels, or shells, labelled using the principal quantum number n (n = 1, 2, 3...).
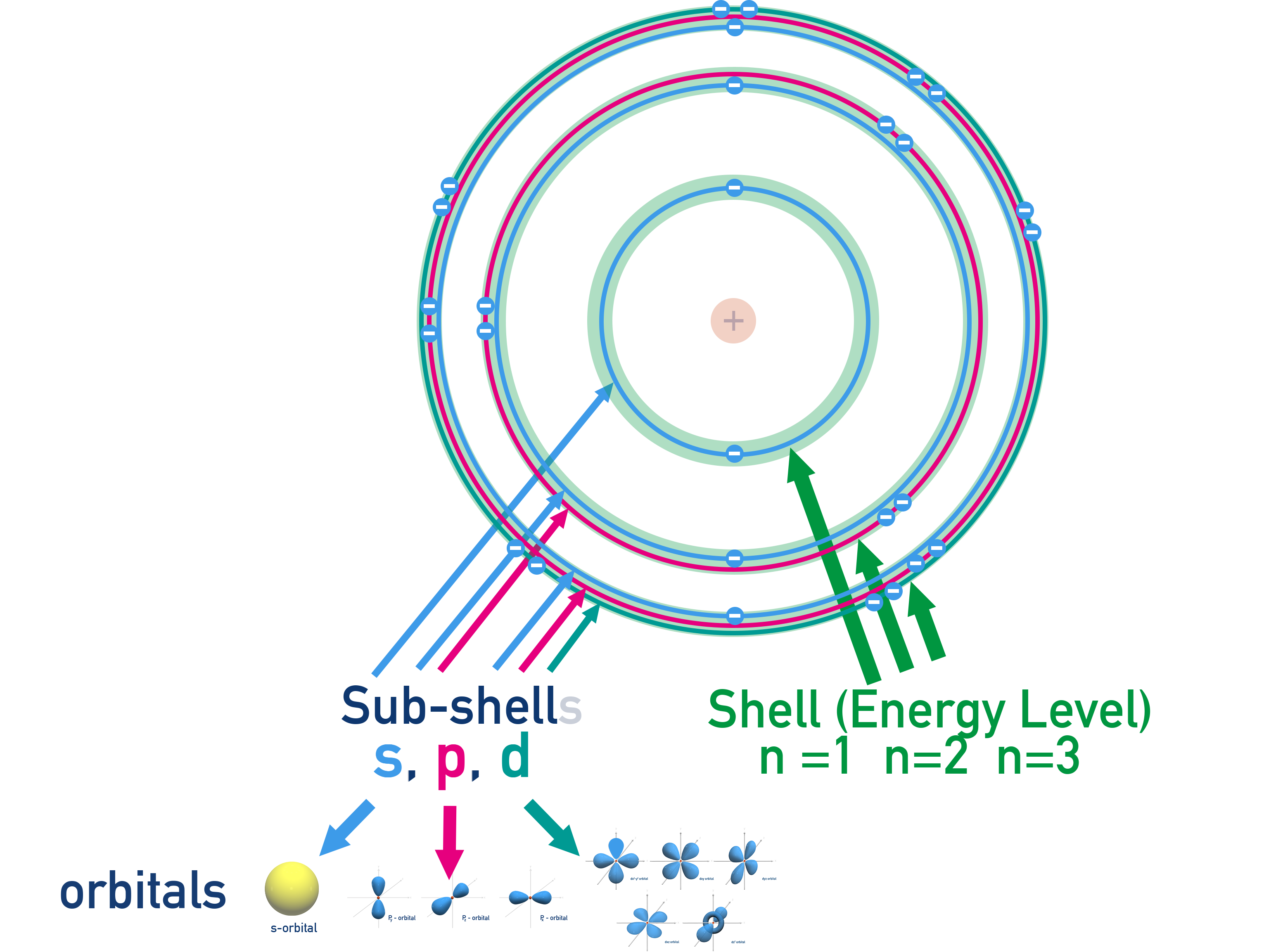
Each shell contains sub-shells: s, p, d (and f from n = 4).
Each sub-shell consists of orbitals, which are regions of space where there is a high probability of finding an electron. Each orbital holds a maximum of 2 electrons with opposite spins.
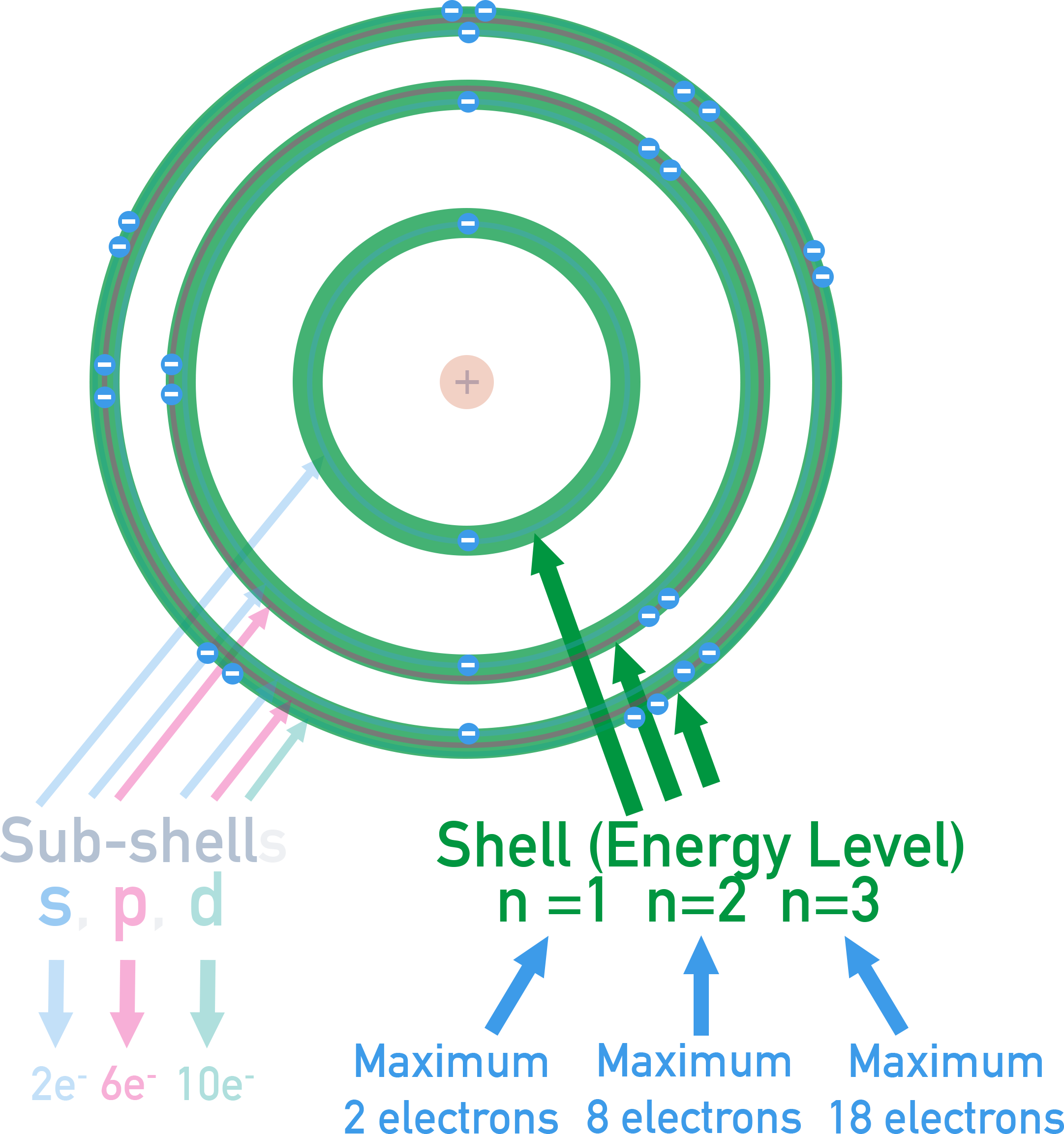
Orbitals in Sub-shells and Their Electron Capacity
- s sub-shell contains 1 orbital → holds 2 electrons
- p sub-shell contains 3 orbitals → holds 6 electrons
- d sub-shell contains 5 orbitals → holds 10 electrons
Energy Order of Sub-shells
Electrons fill sub-shells in order of increasing energy (the Aufbau principle).
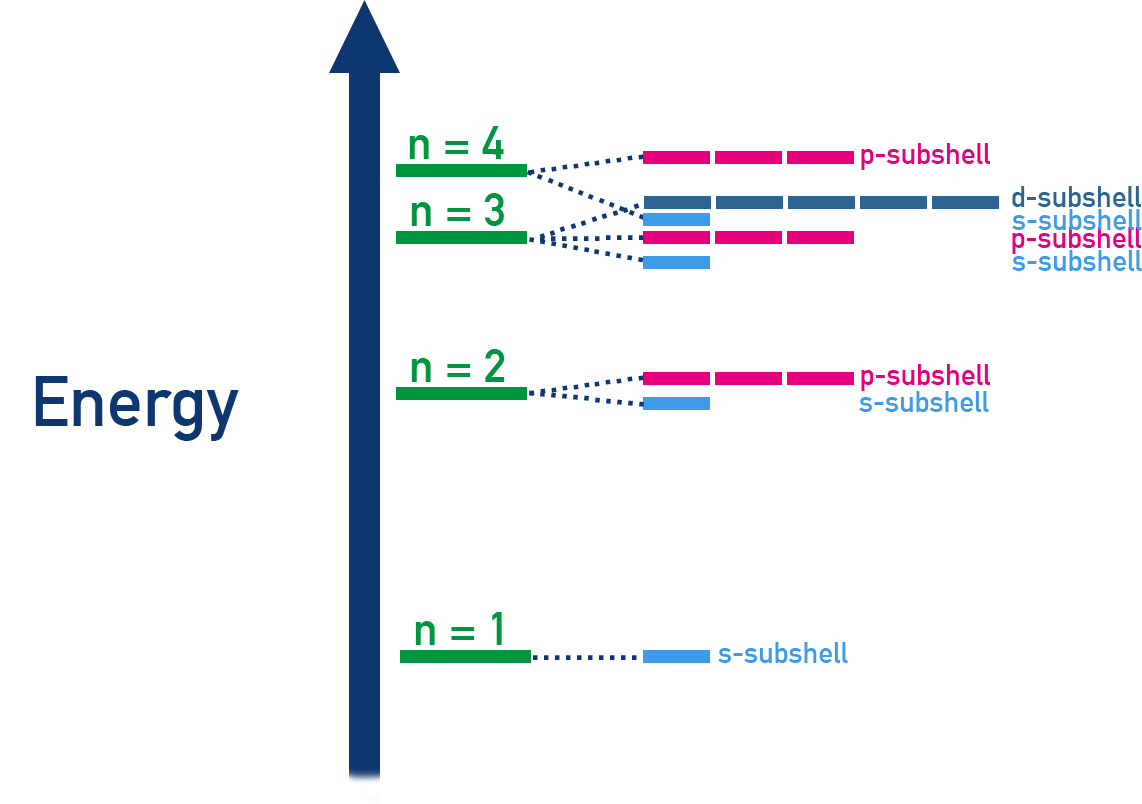
The order up to krypton (Z = 36) is: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p.
Note: The 4s sub-shell is filled before 3d because it is lower in energy.
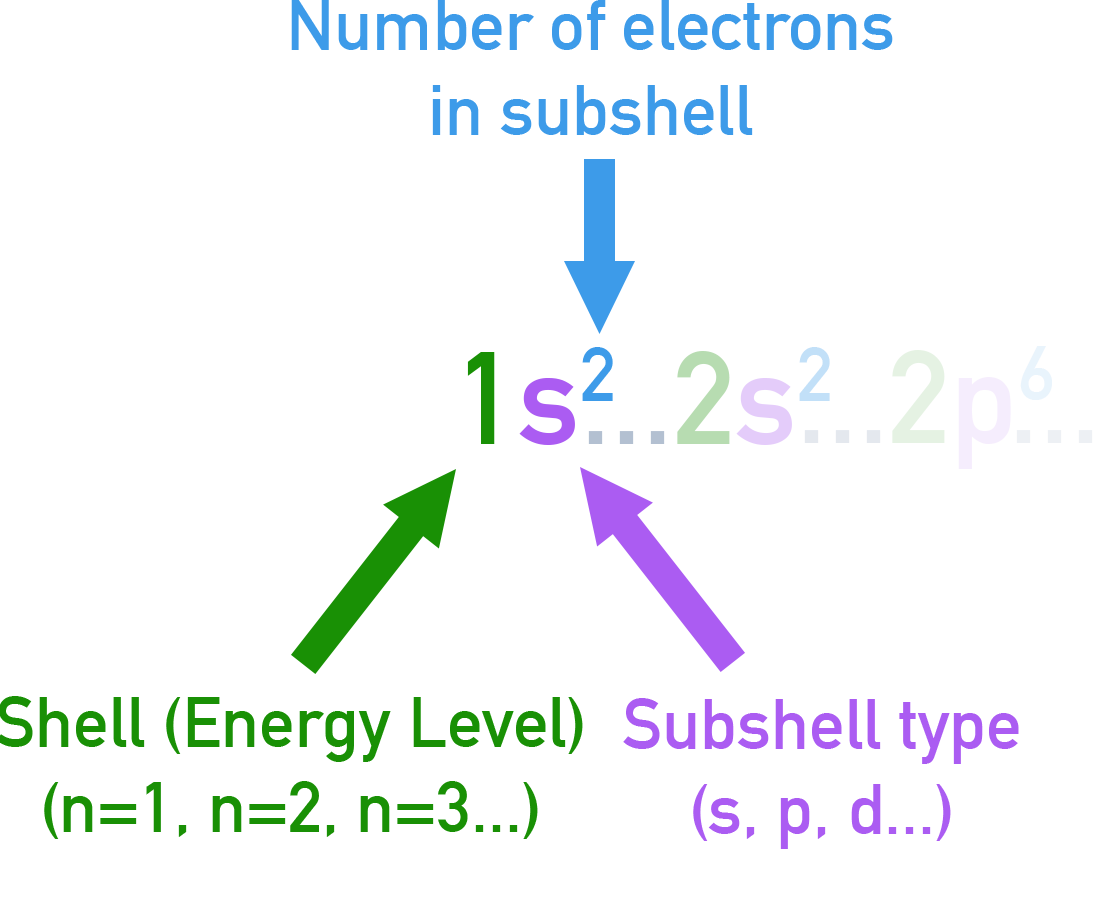
Electron Configurations (Full and Shorthand)
Electron configurations show how electrons are arranged within atoms or ions.

The notation uses a number for the shell (n), a letter for the sub-shell (s, p, d) and a superscript for the number of electrons.
For Example:
- O (Z = 8) → 1s2 2s2 2p4
- Ca (Z = 20) → 1s2 2s2 2p6 3s2 3p6 4s2
Inner electrons can be represented using shorthand notation, refering to a noble gas.
For Example:
- Ca = [Ar] 4s2
- Fe = [Ar] 3d6 4s2 where [Ar] = 1s2 2s2 2p6 3s2 3p6
Energy and Electron Repulsion
Electron configurations are determined by energy levels (electrons fill lower-energy sub-shells first) and electron–electron repulsion (electrons prefer to occupy separate orbitals in a sub-shell before pairing up, as this lowers repulsion). This explains why 4s fills before 3d and also helps explain trends in ionisation energy.
Configurations of Atoms and Ions
In reactions, atoms often gain or lose electrons and as a result their electron configuration changes. Positive ions (cations) lose electrons, starting with the highest energy level. Negative ions (anions) gain electrons into the next available orbital.
Examples Electron configurations for ions:
- Na (Z = 11): 1s2 2s2 2p6 3s1 → Na+ = 1s2 2s2 2p6.
The 3s1 is the highest-energy occupied orbital, meaning this electron is lost first. - Cl (Z = 17): 1s2 2s2 2p6 3s2 3p5 → Cl− = 1s2 2s2 2p6 3s2 3p6.
The empty space in a 3p orbital is the lowest-energy available orbital an electron can go into, meaning the configuration changes from 3p5 to 3p6 and the chlorine atom gains a negative charge.
Electrons-in-Boxes Notation
We can also show electron arrangements using 'box notation' where each orbital is shown as a box with arrows for electrons.
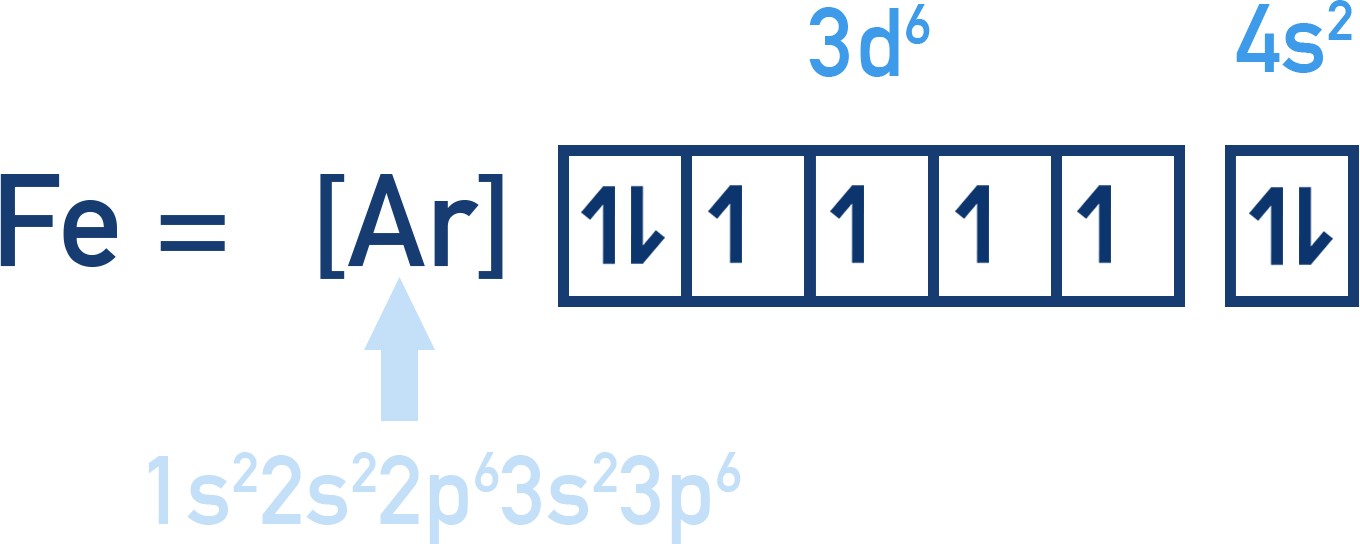
Note that the arrows for electrons in each box (orbital) point in opposite directions, to represent opposite spins.
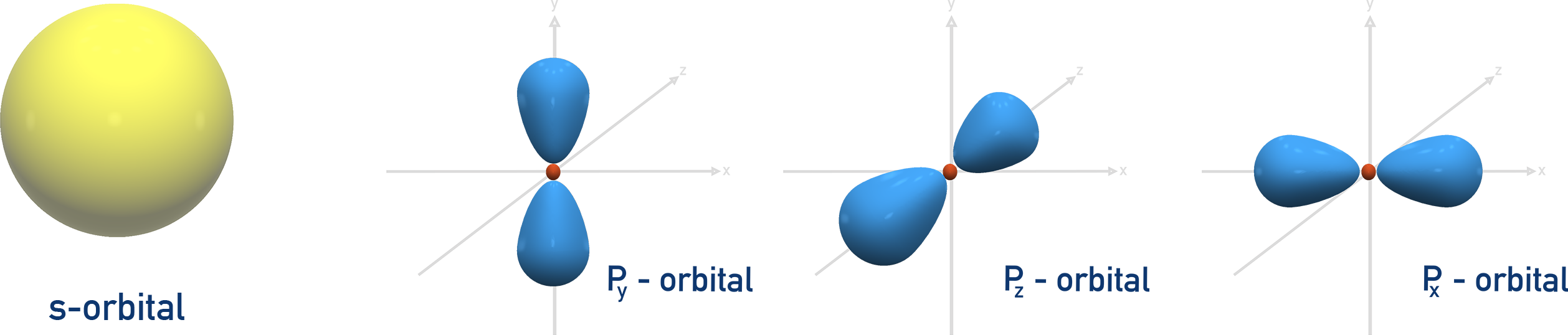
Shapes of s and p Orbitals
s orbital: spherical shape.
p orbital: dumbbell shape, oriented in x, y, and z directions.
These shapes affect how orbitals overlap in bonding.
Free Radicals
A free radical is a species with an unpaired electron. They are highly reactive.
Common Examples:
- Cl• (chlorine radical)
- CH3• (methyl radical).
Radicals and their reactions with alkanes have been covered in more detail here.
Summary
- Electrons occupy orbitals in sub-shells within shells; each orbital holds 2 electrons with opposite spins.
- Sub-shell capacities: s (2), p (6), d (10). Filling order up to 4p follows 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p.
- Electron configurations can be written fully or in shorthand; 4s fills before 3d.
- Electrons prefer to singly occupy orbitals before pairing due to electron–electron repulsion.
- Ion configurations change by loss (cations) or gain (anions) of electrons at the highest/next available energy level.
- s orbitals are spherical; p orbitals are dumbbell shaped; radicals contain an unpaired electron and are highly reactive.
