Atomic Structure and Isotopes, Relative Mass
Quick Notes
- Relative Isotopic Mass
- Mass of one isotope relative to 1/12th the mass of a carbon-12 atom
- Relative Atomic Mass (Ar)
- Weighted mean of all isotopes of an element compared to 1/12th the mass of a carbon-12 atom
- Mass Spectrometry
- Measures relative isotopic masses and their abundances (based on mass to charge, m/z ratio)
- Ar = (Σ isotopic mass × % abundance) ÷ 100
- Relative Molecular Mass (Mr) and Relative Formula Mass
- Mr: sum of Ar values in a molecule
- Relative formula mass: sum of Ar values in an ionic compound
- Both calculated using Ar values from the periodic table
Full Notes
Relative Isotopic Mass
The relative isotopic mass is the mass of a single isotope relative to 1/12th of the mass of a carbon-12 atom.
Relative Atomic Mass (Ar)
Relative atomic mass is the weighted mean of all the isotopes of an element.
It takes into account both the mass and the abundance of each isotope, and it is also relative to 1/12th the mass of a carbon-12 atom.
What Is Mass Spectrometry?
There are several different types of Mass Spectrometry, and they have been explained in more detail here.
Mass spectrometry is a technique used to separate and measure the different isotopes in a sample of an element. It works by ionizing atoms, accelerating them, and then deflecting them based on their mass-to-charge ratio.
Note - you don’t need to know all the details of how a mass spectrometer works however you should be comfortable with the overall idea and purpose of the technique.
Reading a Mass Spectrum
A mass spectrum is a bar graph where:
- The x-axis shows the mass (often the mass number of each isotope).
- The y-axis shows the relative abundance of each isotope, usually as a percentage.
- Each bar represents an isotope. A taller bar means that isotope is more abundant in nature.
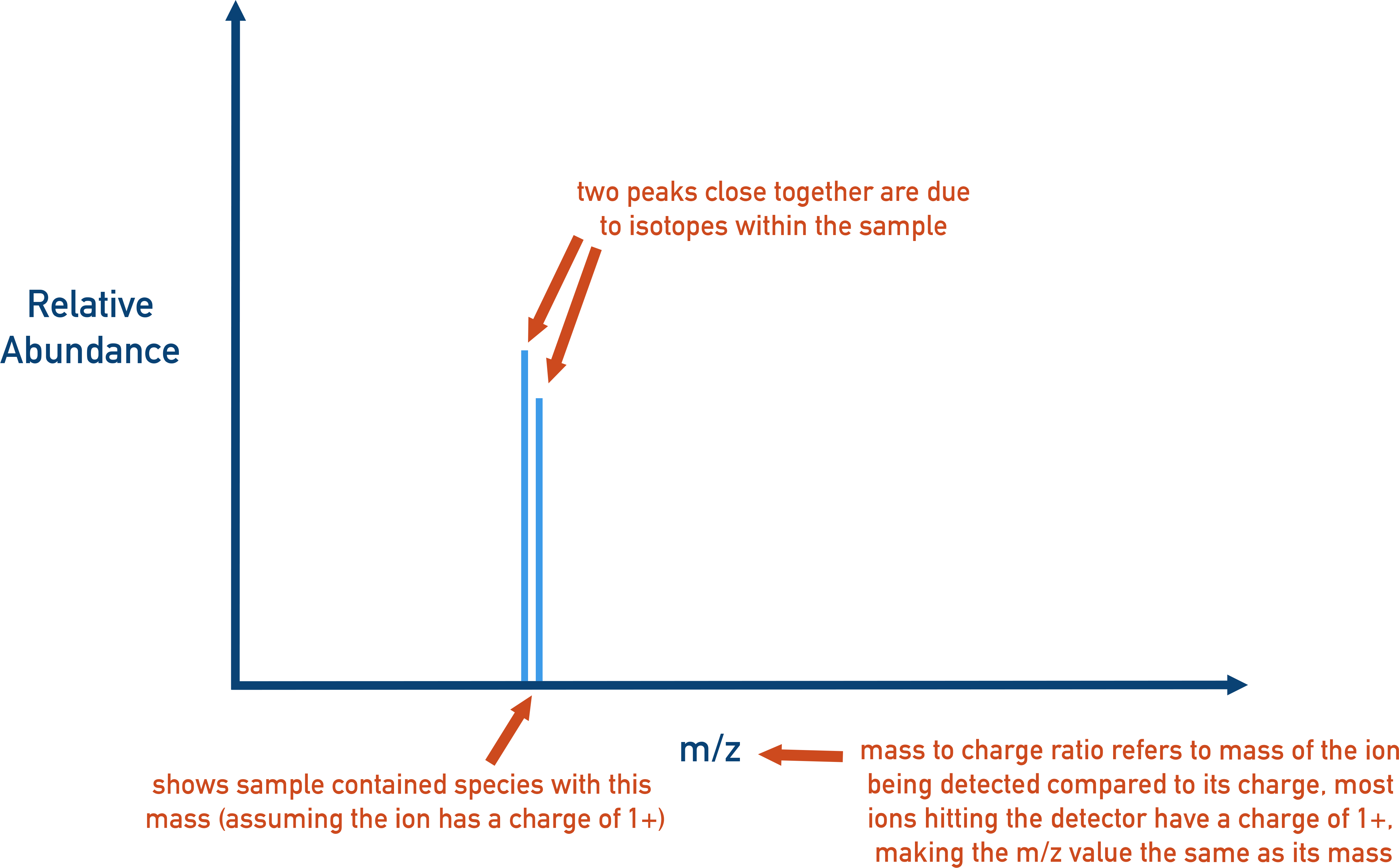

If you are given a mass spectrum with just bars and no percentages, you can treat the bar heights as relative values. Add the total height of all bars, then calculate each bar’s percentage as a fraction of the total.
Calculating Average Atomic Mass
The average atomic mass listed on the periodic table is not a simple average. It is a weighted average that reflects both the mass and natural abundance of each isotope.
It can be calculated using the following formula:
Ar = (Σ (isotopic mass × % abundance)) / 100

To avoid confusion with relative atomic mass, imagine you have 100 atoms of the element.
If isotope A has 75% abundance and isotope B has 25%,
then:
Total mass from A = (mass of A) × 75
Total mass from B = (mass of B) × 25.
Add these to get the combined mass of all 100 atoms
then divide by 100 to find the average mass of one atom – which is the relative atomic mass.
Example Chlorine isotopes
Chlorine has two isotope, giving two peaks in a mess spectra:
- Cl-35 (75%)
- Cl-37 (25%)
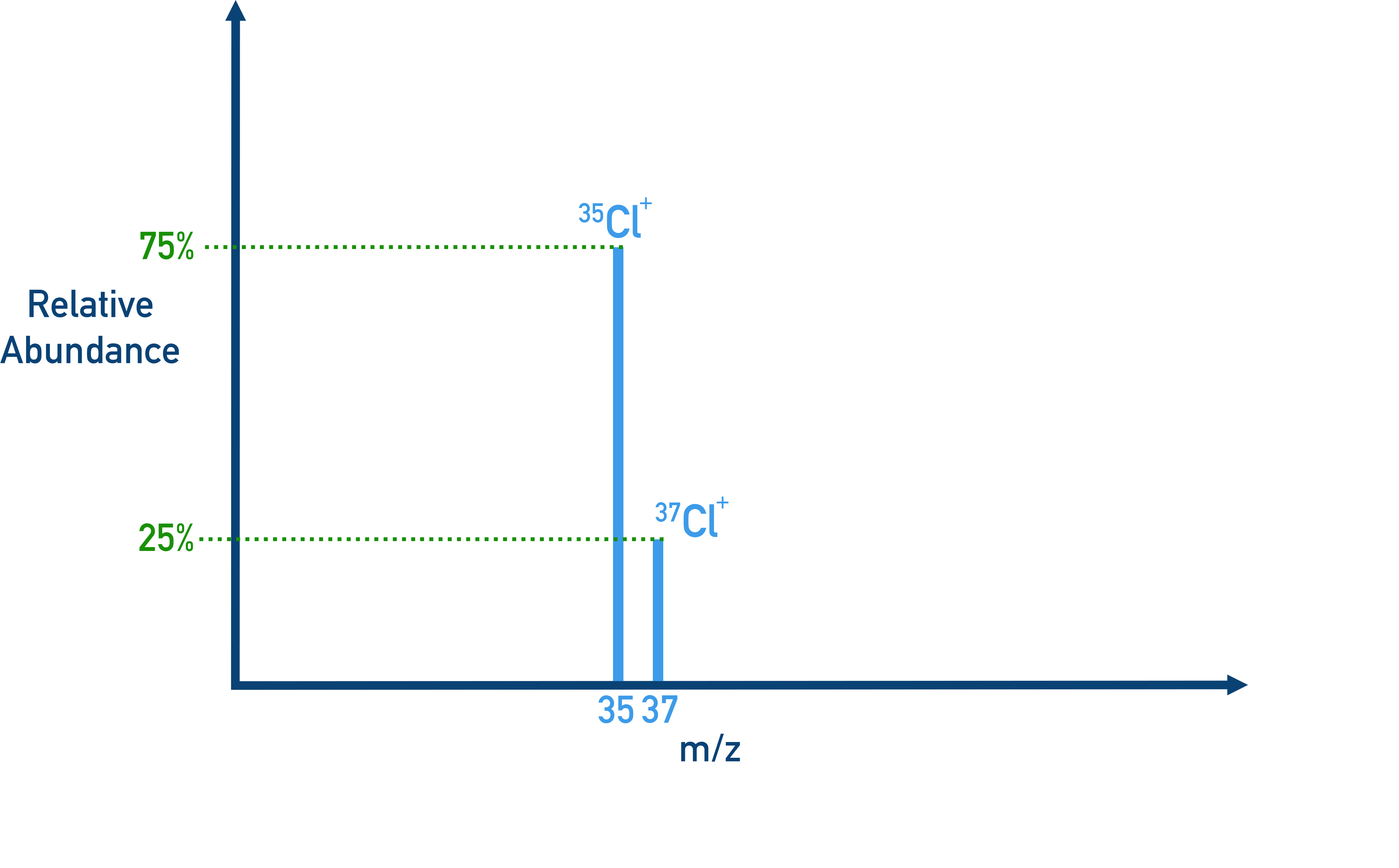
Ar = (35 × 75 + 37 × 25) / 100 = 35.5
Answer: The average atomic mass of chlorine is 35.5
Relative Molecular Mass (Mr) and Relative Formula Mass
Relative molecular mass (Mr) is the sum of the relative atomic masses (Ar) of all atoms in a molecule. It is used for molecular compounds.
Relative formula mass is used for ionic compounds or giant covalent substances. It is calculated the same way, by summing the Ar values of all atoms in the empirical formula.
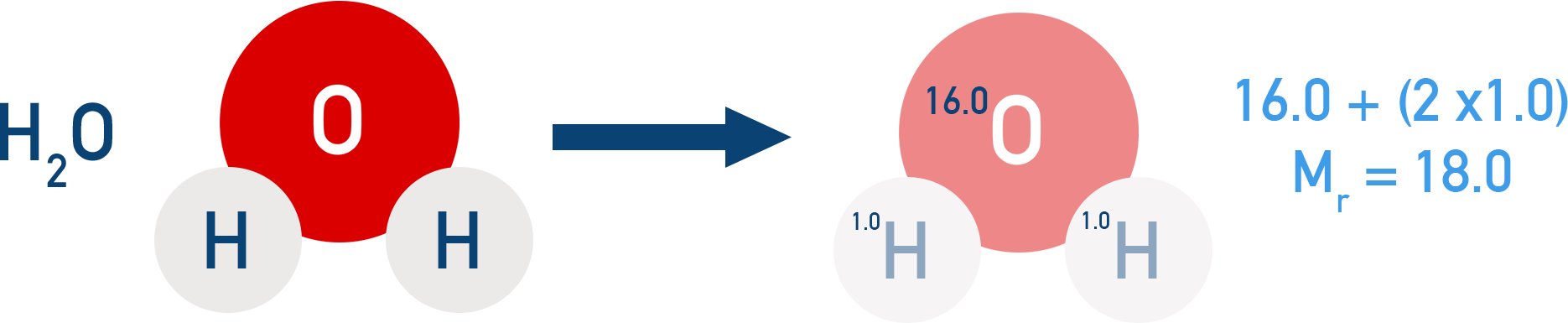
For Example Mr and relative formula mass
Mr of H2O = (2 × 1.0) + (1 × 16.0) = 18.0
Relative formula mass of MgCl2 = 24.3 + (2 × 35.5) = 95.3
Summary
- Relative isotopic mass is for a single isotope, relative to carbon-12.
- Relative atomic mass (A4) is the weighted mean of isotopes, relative to carbon-12.
- Mass spectrometry provides isotopic masses and abundances to calculate Ar.
- Mr is the sum of Ar values in a molecule and relative formula mass applies to ionic compounds.
