Gibbs Free Energy Change
Quick Notes
- Gibbs Free Energy Change (ΔG) determines whether a reaction is feasible (spontaneous).
- Equation for Gibbs Free Energy: ΔG = ΔH − TΔS
-
where:
- ΔG = Gibbs Free Energy change (kJ mol⁻¹)
- ΔH = Enthalpy change (kJ mol⁻¹)
- T = Temperature (K)
- ΔS = Entropy change (J K⁻¹ mol⁻¹)
(converted to kJ K⁻¹ mol⁻¹ by dividing by 1000)
- A reaction is feasible (can happen) when ΔG < 0 (negative).
- Endothermic reactions (+ΔH) can still be feasible if entropy change is positive (+ΔS) and temperature is high.
- Just because a reaction can happen and is feasible doesn’t mean that it will happen - a high activation energy barrier may prevent a feasible reaction from occurring.
Full Notes
Gibbs Free Energy and Reaction Feasibility
Both the enthalpy change (ΔH) and entropy change (ΔS) of a reaction have an impact on whether the reaction can happen. These can be linked, along with temperature (T), by something called Gibbs Free Energy Change (ΔG).
Gibbs Free Energy Change determines whether a reaction can happen spontaneously (is feasible) under standard conditions.
It can be calculated using:
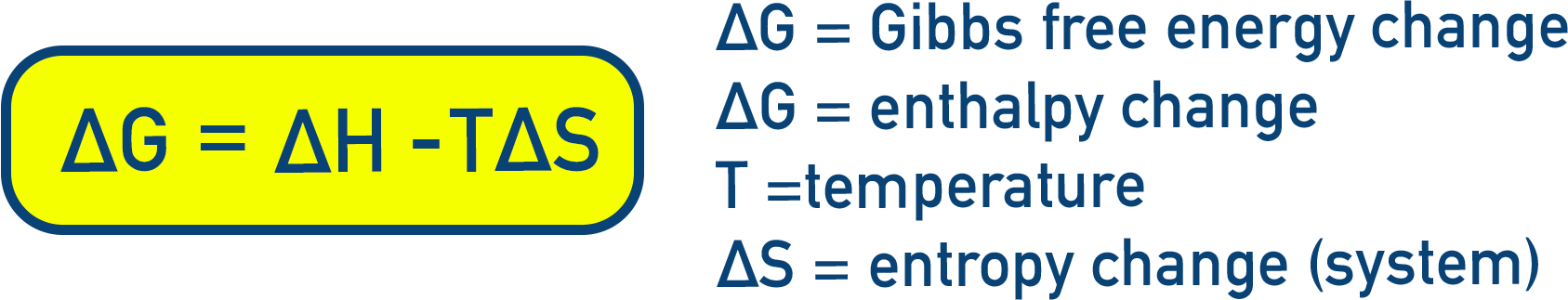
A reaction is feasible if ΔG is negative (ΔG < 0).
Even if ΔG < 0, a reaction may not occur if activation energy is too high.

Remember to check and convert units when using this equation! Entropy change (ΔS) is always given in J per K per mol, whereas Enthalpy change (ΔH) and Gibbs Free Energy Change (ΔG) are given in kJ per mol. Convert entropy to kJ K⁻¹ mol⁻¹ (divide by 1000).
Conditions for Reaction Feasibility
| Condition | Effect on Feasibility |
|---|---|
| ΔH negative, ΔS positive | ΔG is always negative Reaction is always feasible |
| ΔH positive, ΔS negative | ΔG is always positive Reaction is never feasible |
| ΔH negative, ΔS negative | Reaction is feasible at low temperatures |
| ΔH positive, ΔS positive | Reaction is feasible at high temperatures |
Temperature at Which a Reaction Becomes Feasible
To find the minimum temperature (T) at which a reaction is feasible, we can set ΔG = 0:
(If ΔG has to be zero for a reaction to be feasible, then the temperature when ΔG is zero is the minimum that it can be!)
T = ΔH / ΔS
If T is high, the reaction is only feasible at high temperatures.
If T is low, the reaction is feasible at lower temperatures.
Example Calculation: A reaction has the following enthalpy and entropy changes. Find the minimum temperature, T, at which the reaction is feasible.
ΔH = +50 kJ mol⁻¹
ΔS = +100 J K⁻¹ mol⁻¹ (= +0.100 kJ K⁻¹ mol⁻¹)
Use ΔG = ΔH − TΔS and set ΔG = 0:
0 = ΔH − TΔS
T = ΔH / ΔS
T = 50 / 0.100
T = 500 K
The reaction becomes feasible at T ≥ 500 K.
Summary Table: Effect of Temperature on Feasibility
| Condition | Effect on Feasibility |
|---|---|
| ΔH < 0, ΔS > 0 | Always feasible (ΔG < 0) |
| ΔH > 0, ΔS < 0 | Never feasible (ΔG > 0) |
| ΔH < 0, ΔS < 0 | Feasible at low temperatures |
| ΔH > 0, ΔS > 0 | Feasible at high temperatures |
Why Some Feasible Reactions Don’t Happen
Just because a reaction can happen (ΔG is negative) and is feasible doesn’t mean that it will happen - a high activation energy barrier may prevent a feasible reaction from occurring. The rate of reaction may be so slow that the reaction doesn’t happen fast enough to observe.
For example The conversion of diamond into graphite has a negative ΔG, meaning it is thermodynamically feasible. In theory, this process should happen spontaneously.
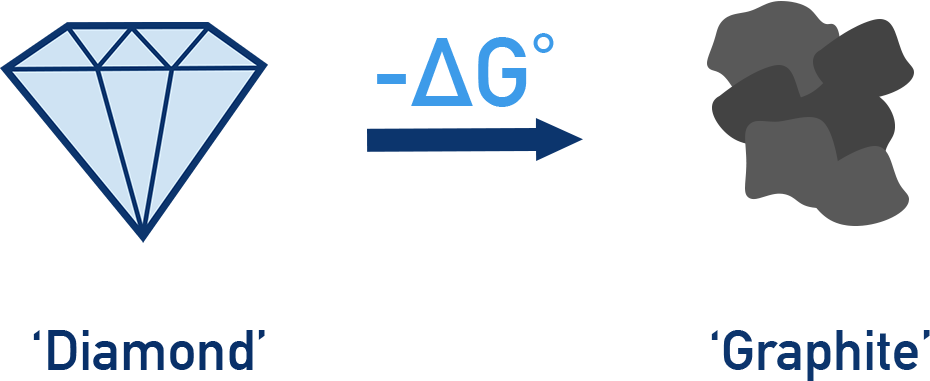
However, in reality, the transformation does not occur noticeably because it has a very high activation energy (Ea). This large energy barrier prevents the reaction from proceeding at a measurable rate under standard conditions.
Summary
- Entropy (S) measures disorder; ΔS tracks change in disorder.
- Use standard entropies (S°) to calculate ΔS: ΣS°(products) − ΣS°(reactants).
- Feasibility is judged with ΔG = ΔH − TΔS (convert ΔS to kJ K⁻¹ mol⁻¹).
- ΔG < 0 → feasible; ΔG > 0 → not feasible.
- Sign rules: (ΔH−, ΔS+) always feasible; (ΔH+, ΔS−) never feasible; (ΔH−, ΔS−) feasible at low T; (ΔH+, ΔS+) feasible at high T.
- Minimum T for feasibility when ΔG = 0 is T = ΔH / ΔS.
- A feasible reaction may be prevented from occuding due to a large activation energy barrier.
