Oxidation Numbers
Quick Notes
- Oxidation number (state): Represents the "charge" an atom would have if the bonding in the compound was fully ionic.
- We assume electrons in bonds 'belong' to the more electronegative atoms, even if no actual electron transfer has occurred.
- Rules for oxidation states:
- Elements in their natural state have an oxidation number of 0.
- Oxygen is usually −2 (except in peroxides where it is −1).
- Hydrogen is usually +1 (except in metal hydrides where it is −1).
- Group 1 metals are +1, Group 2 metals are +2.
- The sum of oxidation states in a neutral compound is 0.
- The sum of oxidation states in an ion equals the charge of the ion.
- Roman numerals in names indicate the oxidation number of an element
(e.g. iron(III) = +3).
Full Notes
Oxidation Numbers
Oxidation numbers help track electron transfer in reactions. It is straightforward to see how atoms have lost or gained electrons when ions get formed, however it can be harder to see how atoms have lost or gained electron density when dealing with molecules.
Example Carbon combustion
Carbon is oxidised to form carbon dioxide when combusted. However, no ions get formed, meaning it isn’t immediately clear how electrons are involved.

To help, we consider each atom to have an ‘imaginary’ charge, described as its oxidation number (or state).
Rules for Assigning Oxidation States
- Uncombined elements (e.g., O2, N2, Fe) have an oxidation state of 0.
- Group 1 metals = +1, Group 2 metals = +2.
- Oxygen is −2, except:
- In peroxides (O22−), oxygen is −1.
- With fluorine (OF2), oxygen is +2.
- Hydrogen is +1, except in metal hydrides (e.g., NaH), where it is −1.
- In a neutral compound, the sum of oxidation states = 0.
- In polyatomic ions, the sum of oxidation states = charge of the ion.
| Element / Case | Oxidation State |
|---|---|
| Uncombined elements (e.g., O2, N2, Fe) | 0 |
| Group 1 metals | +1 |
| Group 2 metals | +2 |
| Oxygen (usual) | −2 |
| Oxygen in peroxides (O22−) | −1 |
| Oxygen in OF2 | +2 |
| Hydrogen (usual) | +1 |
| Hydrogen in metal hydrides (e.g. NaH) | −1 |
| Neutral compound | Sum of oxidation states = 0 |
| Polyatomic ion | Sum of oxidation states = charge of ion |
Using these rules, we can see how carbon gets oxidised from an oxidation state of 0 in C(s) to +4 in CO2(g).
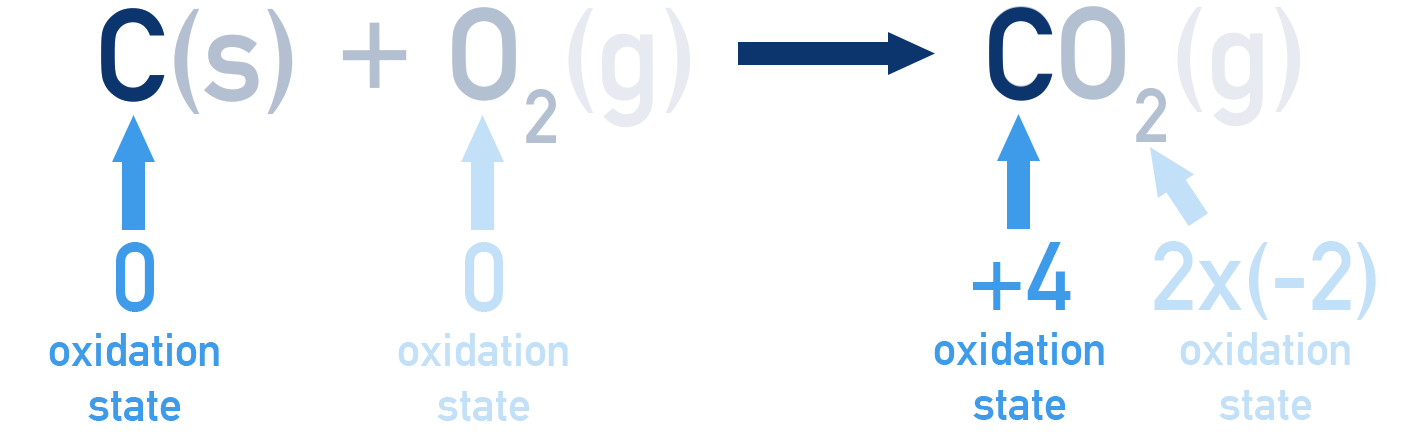
An increase in oxidation number (gets more positive) means oxidation has occurred. A decrease in oxidation number (gets more negative) means reduction has occurred.
Assign oxidation states in H2SO4 (sulfuric acid).
- H = +1 (there are 2 H, total +2).
- O = −2 (there are 4 O, total −8).
- The total charge must be 0, so S must be +6 to balance the equation:
2(+1) + S + 4(−2) = 0 → S = +6.
Roman Numerals in Names
Oxidation numbers are shown in Roman numerals in the names of compounds, particularly for transition metals and other elements with variable oxidation states.
Examples
- FeCl2 is named iron(II) chloride because Fe is +2
- FeCl3 is named iron(III) chloride because Fe is +3
- MnO4− is named manganese(VII) oxide because Mn is +7
Summary
- Oxidation numbers are imaginary charges assigned to atoms to track electron transfer.
- Rules include: elements = 0, oxygen = −2 (with exceptions), hydrogen = +1 (with exceptions), group 1 = +1, group 2 = +2.
- The sum of oxidation states = 0 in neutral compounds and = charge in ions.
- Oxidation = increase in oxidation number, reduction = decrease in oxidation number.
- Roman numerals indicate oxidation numbers in compound names.
