Standard Electrode Potentials
Quick Notes
- A standard electrode potential (E°) is the potential of a half-cell compared to the standard hydrogen electrode (SHE) under standard conditions.
- Standard conditions:
- 298 K (25°C)
- 100 kPa pressure
- 1.00 mol dm⁻³ ion concentrations
- Standard Hydrogen Electrode (SHE) is a reference electrode with E° = 0.00V.
- Electrode Potentials (E°) are measured by connecting a half-cell to the SHE using a salt bridge and voltmeter.
- Different types of half-cell:
- Metal/ion half-cell: metal in a solution of its own ions.
- Ion/ion half-cell: platinum electrode with two ions of the same element.
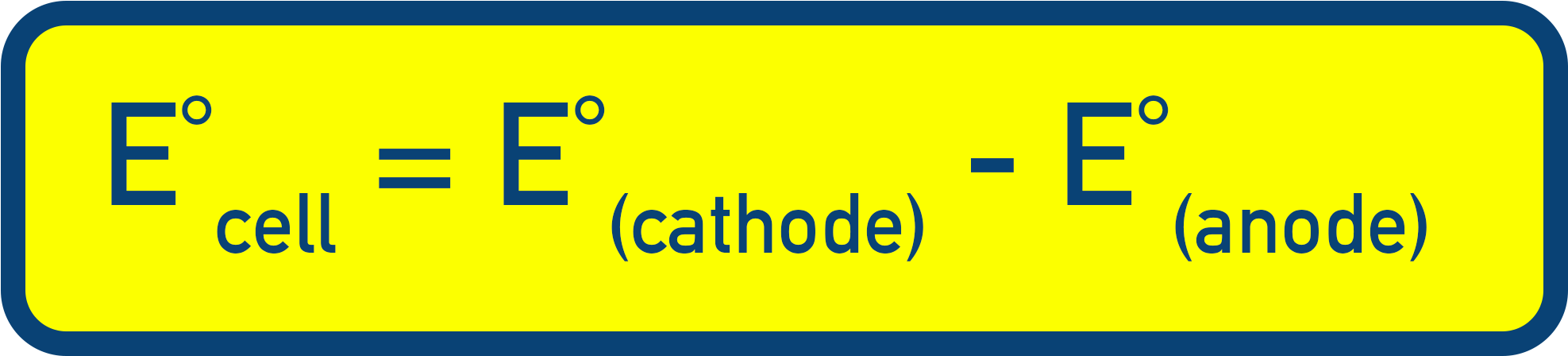
- Standard Cell Potential (E°cell)
- Overall voltage from two half-cells under standard conditions.
- E°cell = E°(cathode) − E°(anode).
- Cathode = more positive E°, anode = more negative E°.
- Electron Flow and Reaction Feasibility
- Electrons flow from anode to cathode.
- Positive E°cell = forward reaction feasible.
- Negative E°cell = reverse reaction favoured.
- Reactivity Trends from E° Values
- Higher E° = stronger oxidising agent.
- Lower E° = stronger reducing agent.
- E°cell predicts feasibility: a positive value suggests a reaction is feasible.
Full Notes
Electrochemical cells and standard electrode potentials have been covered in more detail here.
This page is just what you need to know for OCR (A) A-Level :)
Every chemical species has a tendency to either gain or lose electrons. We can measure this tendency using something called electrode potentials, E°.
Electrode potentials tell us how easily a species can be reduced (gain electrons) or oxidised (lose electrons).
- A more positive electrode potential means the species is more likely to gain electrons (be reduced).
- A more negative electrode potential means the species is more likely to lose electrons (be oxidised).
Half-Cells
Simple half-cells are made of a metal solid placed into a solution that contains ions of the metal. The metal solid is called an electrode and the solution it is in an electrolyte.
A redox equilibrium is established between the ions in the electrolyte and the solid metal electrode.
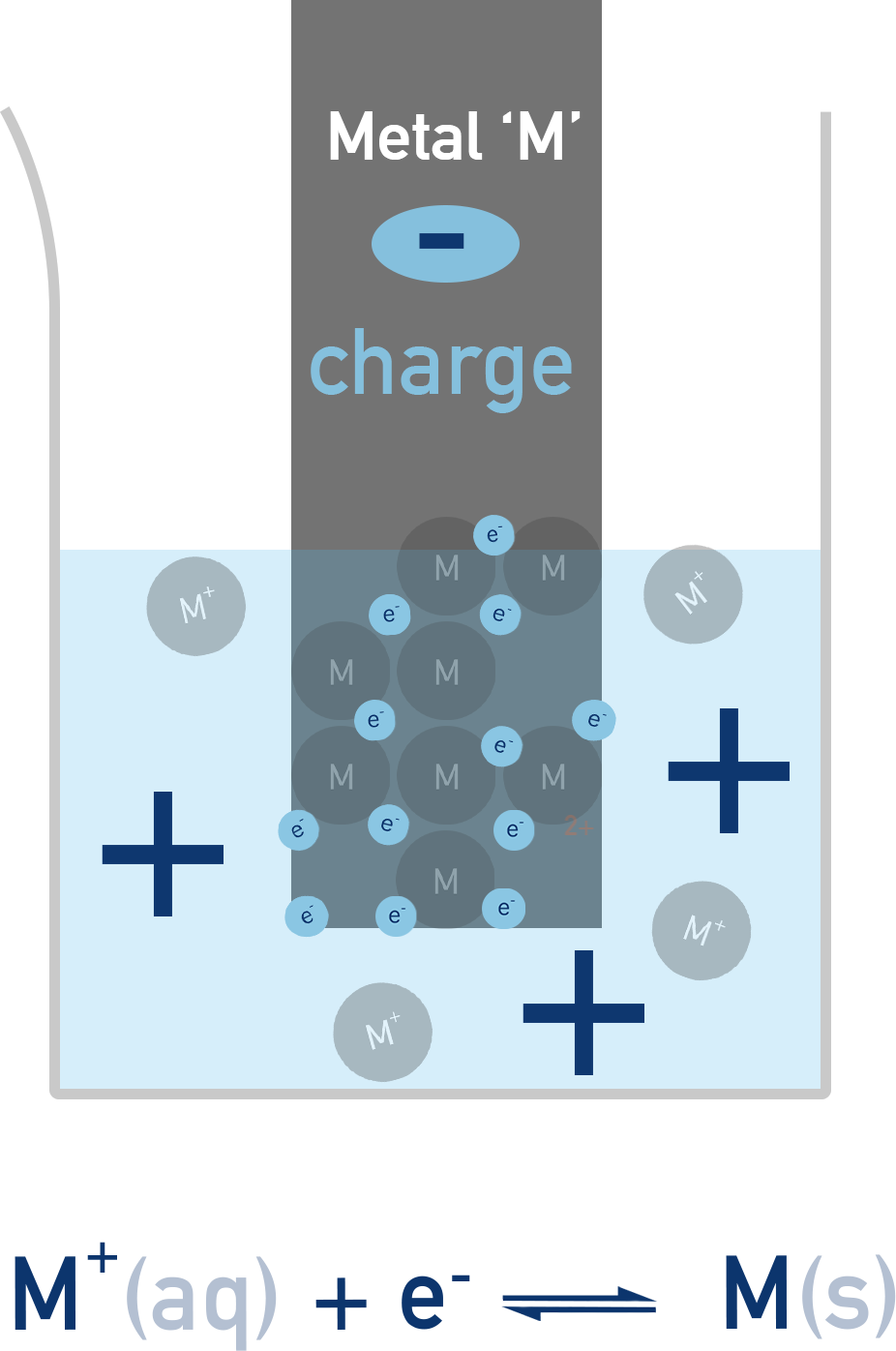
This sets up a potential difference between the metal and the solution, that depends on how far the equilibrium lies to the left or right. This potential difference is the electrode potential.
The electrode potential can’t be measured directly however we can compare different electrode potentials for different half-cells by connecting them to a reference half-cell and measuring the potential difference each time.
The reference used is the Standard Hydrogen Electrode (SHE).
The Standard Hydrogen Electrode (SHE)
The Standard Hydrogen Electrode (SHE) is used as the universal reference point and consists of:
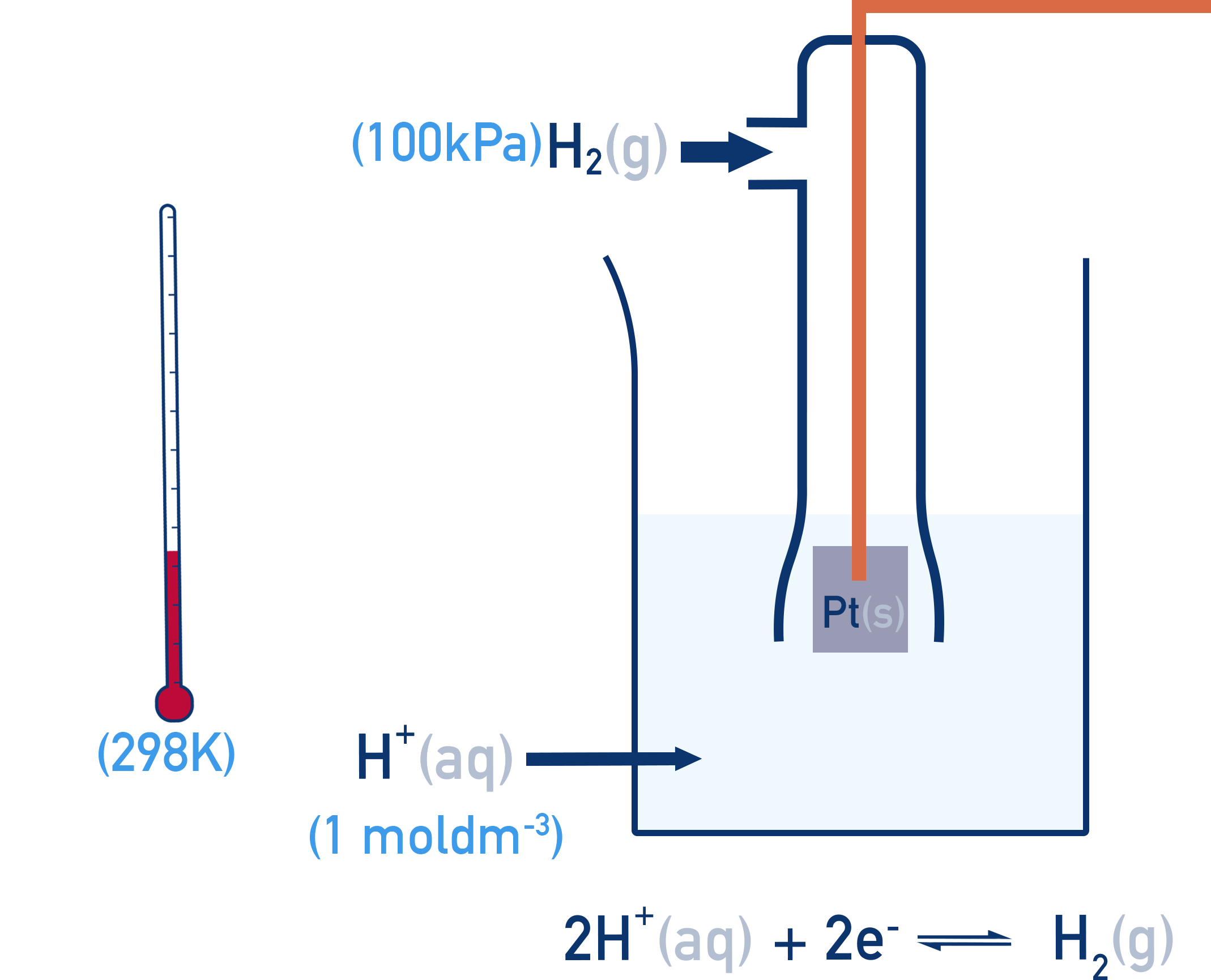
- Hydrogen gas at 100 kPa
- Bubbled over a platinum electrode
- In 1.00 mol dm⁻³ H⁺ solution
- At 298 K (25°C)
All standard electrode potentials (E°) are measured under these conditions and describe the potential of a half-cell compared to the Standard Hydrogen Electrode.
The Standard Hydrogen Electrode is assigned a potential of 0.00 V. All this means is that when two standard hydrogen electrodes are connected together, the potential difference is 0.00 V.
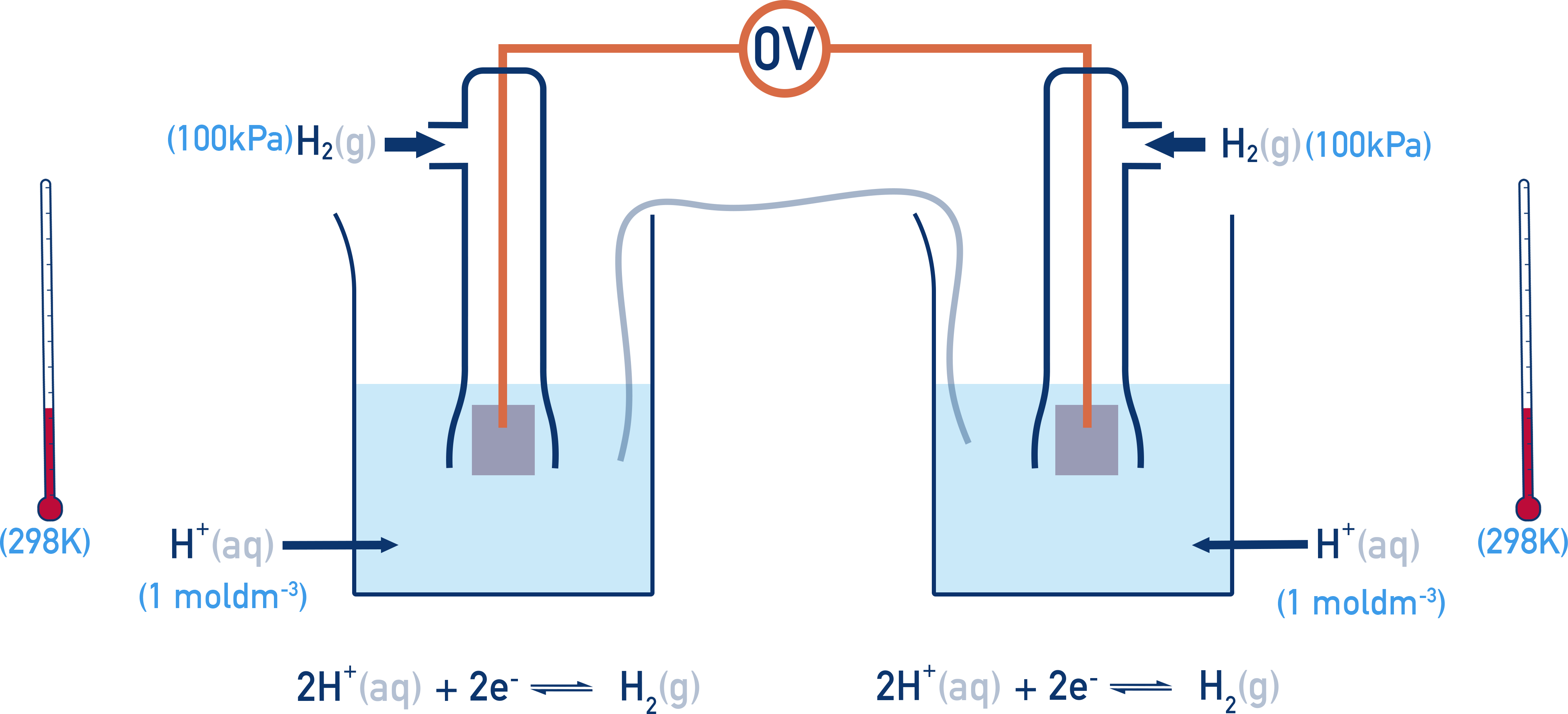
If the right hand half-cell is now changed, a potential difference (voltage) is measured and is called the standard electrode potential (E° value) of the right hand half-cell.
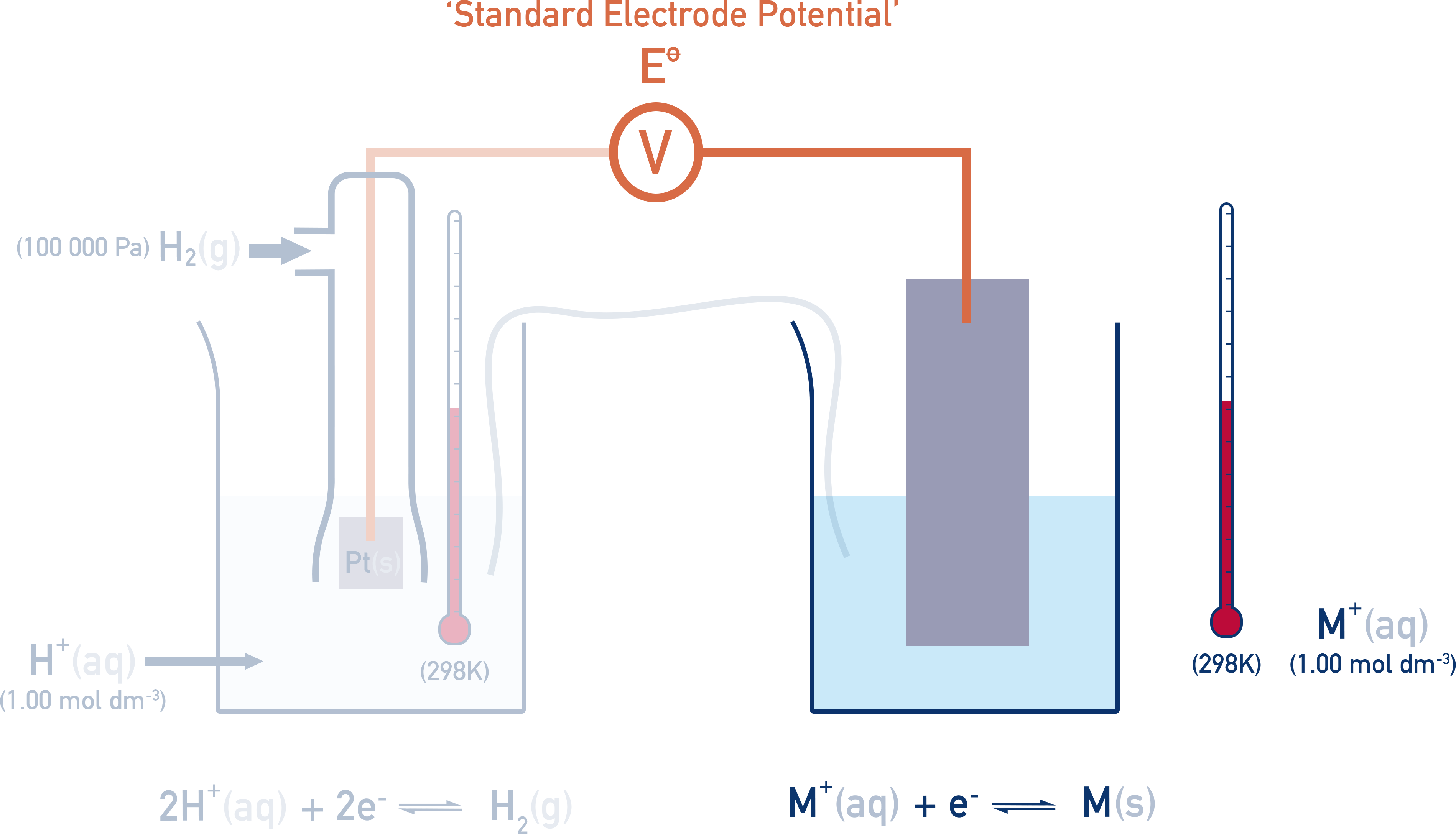
The temperature, concentration and pressure (for gases) must be the same as the standard hydrogen electrode (1.00 mol dm⁻³, 298 K and 100 kPa of pressure), otherwise positions of equilibrium in each half-cell will be affected and comparisons between measured potentials won’t be representative.
Standard electrode potentials are often put into a table called the electrochemical series (shown below).
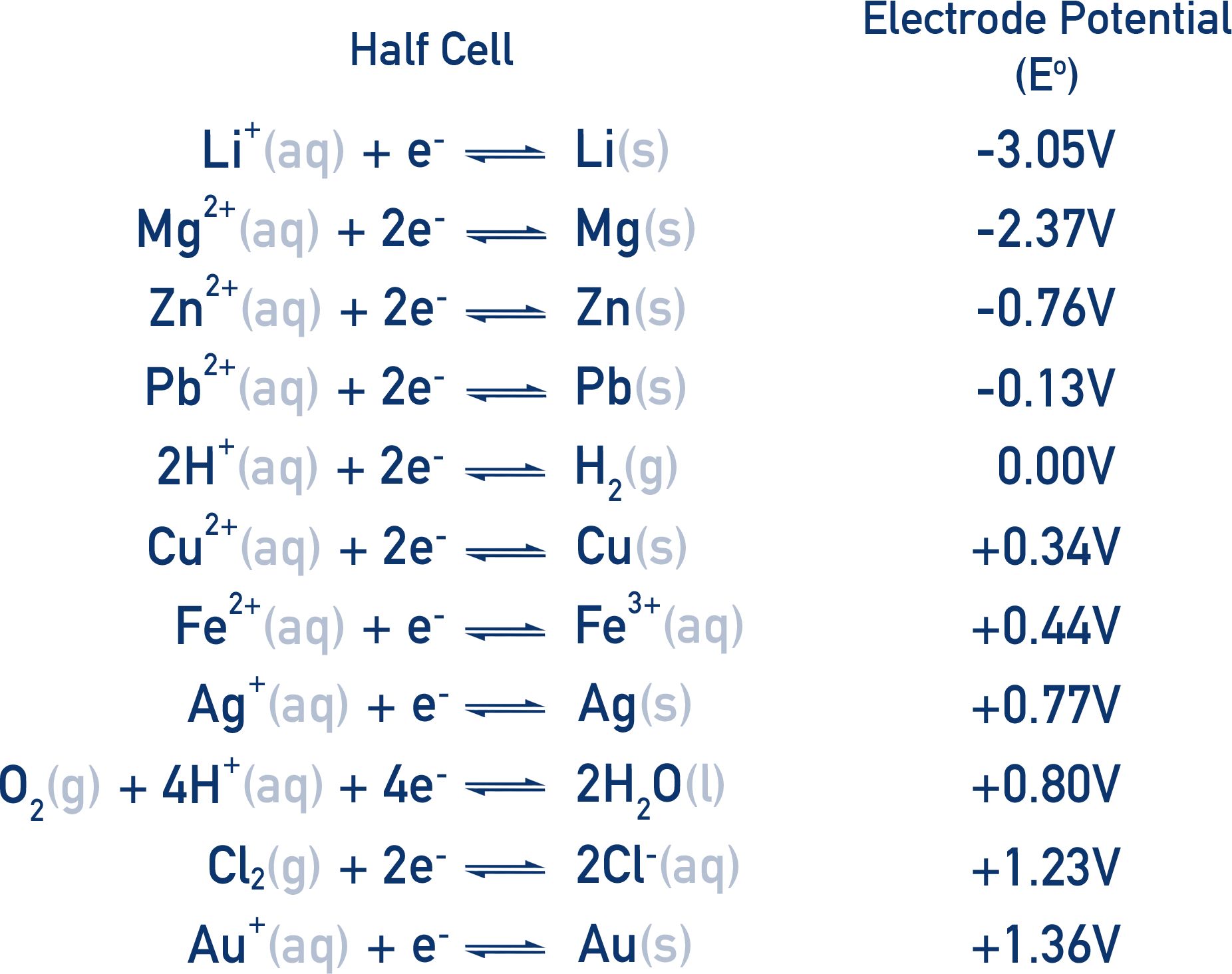
The more positive the E°, the more likely a species in the half-cell is to be reduced.
The more negative the E°, the more likely a species in the half-cell is to be oxidised.
Definition of Standard Electrode Potential (E°)
The standard electrode potential, E°, measures the tendency of a species to gain electrons under standard conditions. It is always measured relative to the standard hydrogen electrode (SHE), which is assigned a potential of 0.00 V.
Standard conditions:
- Temperature = 298 K
- Pressure = 100 kPa (for gases)
- Ion concentration = 1.00 mol dm⁻³
A more positive E° indicates a greater tendency to be reduced (gain electrons).
Types of Electrodes
Half-cells can be made in different ways depending on the species involved:
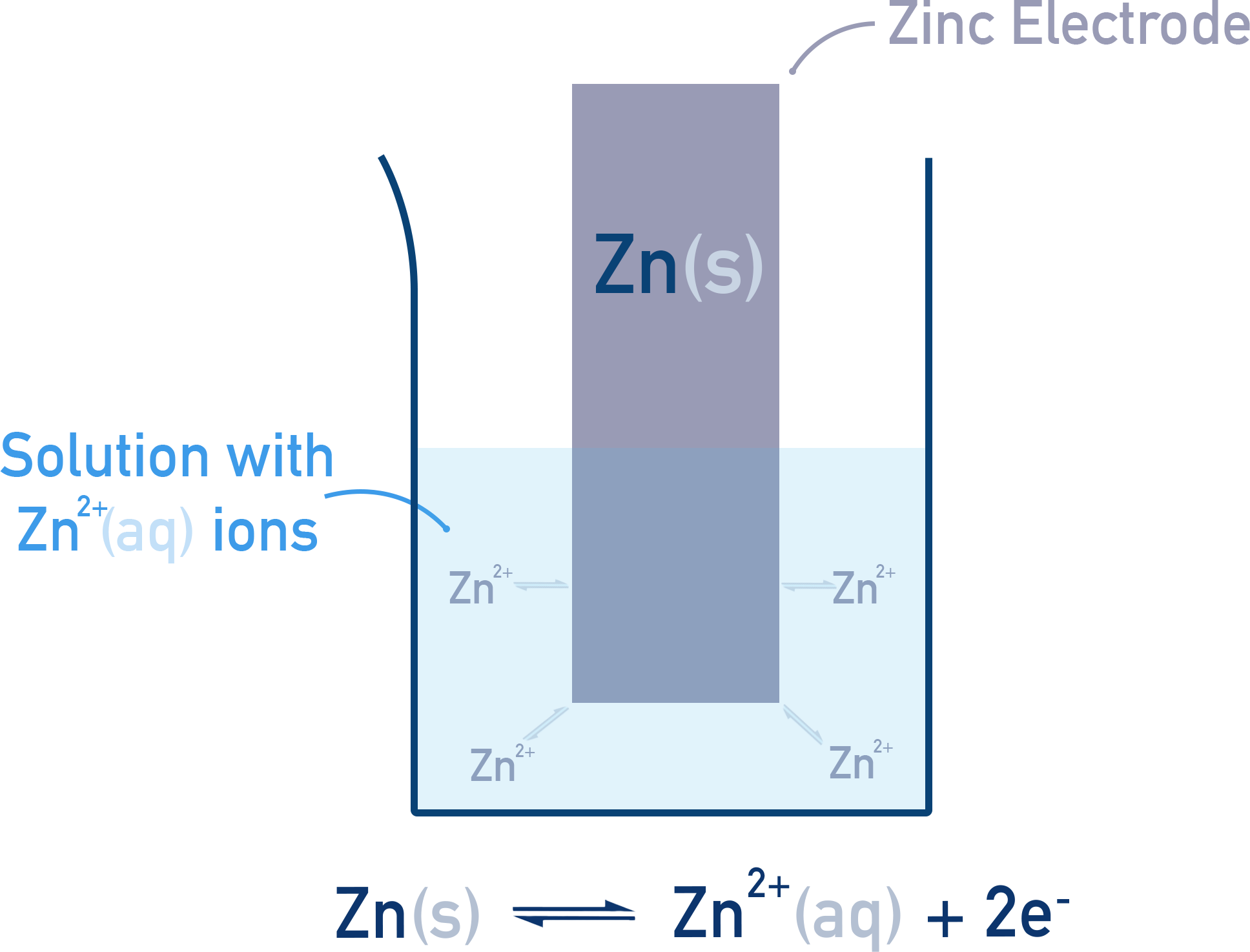
Metal or Non-Metal Half-Cells:
Example: A zinc rod in Zn²⁺ solution.
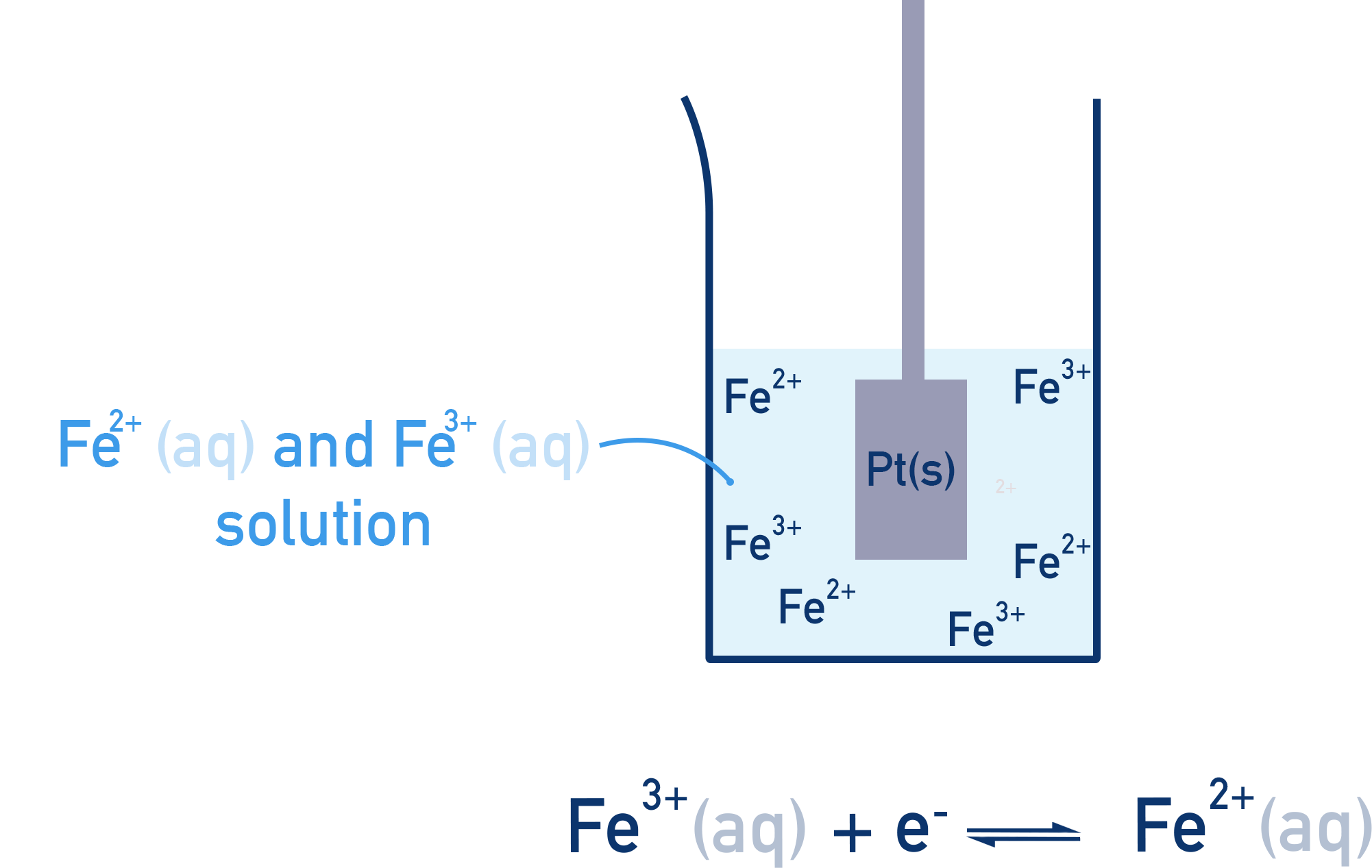
Different Oxidation States of the Same Element:
Example: A solution containing both Fe³⁺ and Fe²⁺ ions (with a platinum electrode).
Importance of Standard Conditions
Deviation from standard conditions (e.g. changes in temperature, pressure, or concentration) can affect measured potentials.
- If concentrations change, then the actual potentials of each half-cell will change.
- Increasing the concentration of oxidised species in the half-cell makes E more positive.
- Increasing the concentration of reduced species in the half-cell makes E more negative.
This affects how easily redox reactions occur under non-standard conditions.
Standard Cell Potential (Ecell)
The standard cell potential is the overall voltage produced when two half-cells are connected under standard conditions.
It’s calculated by:

Note:
- The cathode is the half-cell where reduction happens.
- The anode is the half-cell where oxidation happens.
Meaning you can also write this as:

Determine the Ecell when the following two half-cells are connected together:
- Zn2+(aq) + 2e− ⇌ Zn(s) E° = –0.76 V
- Cu2+(aq) + 2e− ⇌ Cu(s) E° = +0.34 V
Here, Cu2+/Cu will be the cathode (reduction), and Zn2+/Zn will be the anode (oxidation).
E°cell = (+0.34) − (−0.76) = +1.10 V
Predicting Thermodynamic Feasibility
You can predict whether a redox reaction is feasible (able to happen) using the calculated standard cell potential:

If E°cell is positive, the reaction is thermodynamically feasible under standard conditions.
However, a feasible reaction may not happen due to kinetic barriers like high activation energy.

Remember standard electrode potentials apply only under standard conditions. If conditions change, the actual Ecell may differ from E°cell (calculated using standard electrode potentials), which can explain why a reaction predicted to be feasible doesn’t occur in practice.
Summary
- E° compares a half-cell to the standard hydrogen electrode (SHE) under standard conditions.
- SHE: 0.00 V at 298 K, 100 kPa, 1.00 mol dm⁻³ H⁺ with H₂ gas and Pt electrode.
- More positive E° = stronger tendency to be reduced and a more negative E° = stronger tendency to be oxidised.
- Half-cells include metal/ion and ion/ion systems (inert Pt electrode).
- Changing temperature, pressure or concentrations shifts measured potentials away from standard values.
- E°cell = E°(cathode) − E°(anode).
- A positive Ecell value means reaction is feasible
- Feasible reactions may still be unable to occur due to high activation energy barriers
