Atomic and Molecular Masses
Learning Objective: Understand how atoms and molecules are assigned mass, and how to calculate them for elements, compounds, and ionic formulas.
Quick Notes:
- Atomic mass is the mass of a single atom, expressed in atomic mass units (amu).
- 1 amu = 1/12 the mass of one carbon-12 atom.
- Most elements have isotopes, so we use average atomic mass based on relative abundance.
- Molecular mass = sum of atomic masses of all atoms in a molecule (used for covalent compounds such as H2O, CO2).
- Formula mass is used for ionic compounds like NaCl, since they don’t form molecules.
- All masses are relative to the carbon-12 standard.
Full Notes:
1.7.1 Atomic Mass
Atoms are extremely small and their actual masses in grams are minuscule. For example, one atom of hydrogen has a mass of approximately 1.6736 × 10⁻²⁴ g.
To simplify calculations, scientists use a relative scale to compare atomic masses.
Definition:
The mass of an atom is expressed in atomic mass units (amu), where:
1 amu = (1/12) × mass of one carbon-12 atom
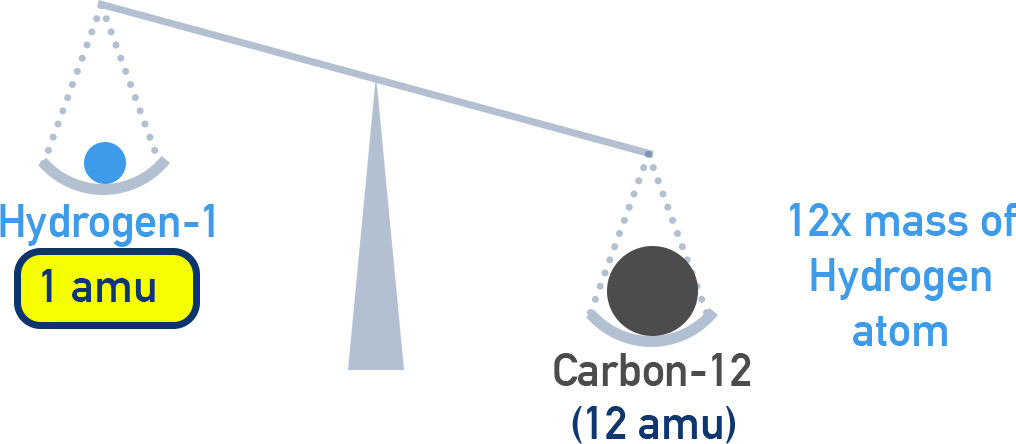
1 amu therefore equals approximately the mass of one atom of H-1 (which is 1/12 mass of a C-12 atom).
This is the international standard, chosen because the carbon-12 isotope is stable and easily measurable.
Examples:
- Hydrogen = 1.008 amu
- Carbon = 12.00 amu
- Oxygen = 16.00 amu
These values are determined using mass spectrometry, which gives highly accurate ratios.
Note: 1 amu ≈ 1.66056 × 10⁻²⁴ g
1.7.2 Average Atomic Mass
Most elements exist as a mixture of isotopes. The atomic mass listed on the periodic table is not for a single atom, but the weighted average of all naturally occurring isotopes.
Formula:
Average atomic mass
= (% of isotope A × mass of A) + (% of isotope B × mass of B) + …
Example Chlorine

³⁵Cl (75.77%) → 34.9689 amu
³⁷Cl (24.23%) → 36.9659 amu
Average atomic mass = (75.77 × 34.9689 + 24.23 × 36.9659) / 100 = 35.5 amu
This is the value shown for chlorine on the periodic table.
1.7.3 Molecular Mass
This refers to the sum of the atomic masses of all the atoms in a molecule.
Formula:
Molecular mass = Σ (atomic mass of each atom in the molecule)
Example Water (H₂O)
= 2 × H (1.008) + 1 × O (16.00)
= 2.016 + 16.00 = 18.02 amu
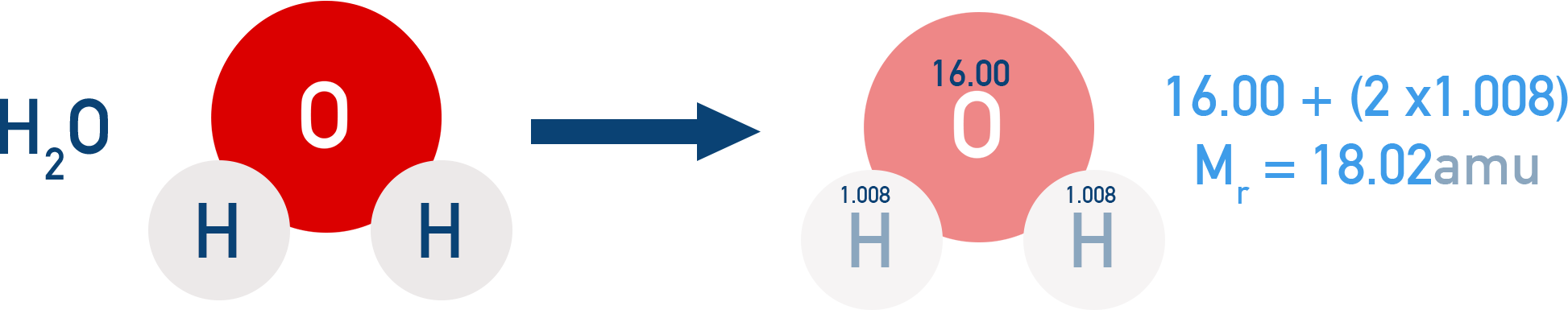
This method applies to covalent compounds, where molecules exist as discrete units.
1.7.4 Formula Mass
Some compounds, especially ionic compounds, do not exist as individual molecules. Instead, they form three-dimensional lattice structures made of ions.
So instead of molecular mass, we calculate the formula mass, based on the simplest repeating formula unit.
Definition:
Formula mass = Sum of atomic masses of ions present in a formula unit.
Example Sodium chloride (NaCl)
Na = 22.99 amu
Cl = 35.45 amu
Formula mass = 22.99 + 35.45 = 58.44 amu
Even though NaCl does not exist as molecules, we can still use its chemical formula to calculate mass for stoichiometric purposes.

Don’t confuse molecular mass (used for molecules like water, CO₂) with formula mass (used for ionic compounds like NaCl, CaCO₃). Always identify whether the compound is covalent or ionic before calculating.
Summary
- Atomic mass is expressed in amu based on the carbon-12 scale.
- Average atomic mass accounts for natural isotopic abundance.
- Molecular mass applies to covalent compounds with discrete molecules.
- Formula mass is used for ionic compounds forming lattice structures.
- Mass spectrometry determines atomic and molecular masses precisely.
