Thermodynamic Terms
Quick Notes
- System: The part under study
- Surroundings: Everything else
- Types of systems:
- Open – exchange of matter and energy
- Closed – exchange of energy only
- Isolated – no exchange of matter or energy
- Work and heat are path-dependent functions
- State of a system defined by variables: pressure, volume, temperature, composition
Full Notes
Introduction
Thermodynamics is the study of energy changes during physical and chemical processes.
To analyse such changes, we define reacting particles as a system and surrounding particles or materials as the surroundings. In chemistry, this helps us track how energy is transferred or transformed during reactions.
5.1.1 The System and the Surroundings
It is really important to understand what we are referring to when we consider energy changes:
- System: The specific part of the universe under thermodynamic investigation.
- For example When heating water in a beaker, the water is the system.
- Surroundings: Everything external to the system that can exchange energy or matter with it.
- Boundary: The real or imaginary surface separating the system from its surroundings.
- The universe = system + surroundings
5.1.2 Types of the System
Thermodynamic systems are classified based on the exchange of matter and/or energy:
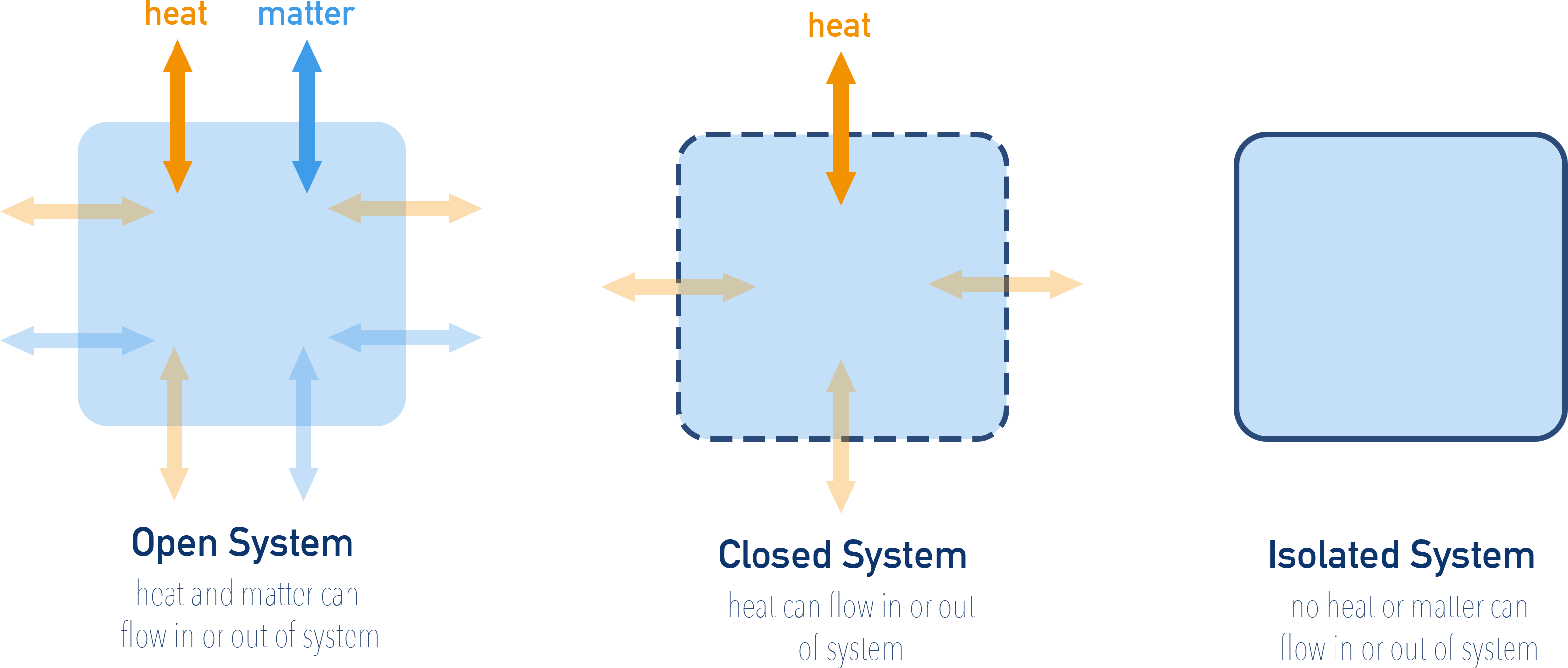
- Open System: Both matter and energy can be exchanged with surroundings.
- Closed System: Only energy is exchanged; matter remains constant.
- Isolated System: Neither energy nor matter is exchanged.
5.1.3 The State of the System
The state of a thermodynamic system is defined by certain measurable properties known as state variables or state functions – pressure (P), volume (V), temperature (T), and composition.
A change in state is described by how these variables change.
When a system undergoes a change, energy transfer may occur in two forms: work and heat.
Work (w)
- Energy transferred when an object is moved by a force.
- Commonly seen in expansion or compression of gases.
- Work is a path-dependent quantity – depends on how the process is carried out.
Heat (q)
- Energy transferred due to temperature difference between system and surroundings.
- Also path-dependent.
- Like work, heat alters the internal energy of the system.
The General Case
- Energy can enter or leave the system via both heat and work.
- These are not properties of the system, but rather of the process.
- When either heat or work is supplied to a system, its internal energy changes.
5.1.4 Internal Energy as a State Function
In thermodynamics, we define a system’s total energy as its internal energy (U).
This includes all forms of microscopic energy – chemical, electrical, mechanical, and others – present within the system.
The internal energy may change if:
- Heat is transferred into or out of the system,
- Work is done on or by the system,
- Matter enters or leaves the system.
Work and Internal Energy – The Adiabatic Approach
To understand how work affects internal energy, consider an adiabatic system – a system insulated from its surroundings so no heat is exchanged. A thermos flask or an insulated beaker would be an example.
An adiabatic process is one where no heat (q = 0) is exchanged between the system and surroundings.
- Initial state A: temperature = TA, internal energy = UA
- Final state B: temperature = TB, internal energy = UB
- Change in internal energy: ΔU = UB − UA
Example 1: Mechanical Work
Stirring water using a stirrer adds 1 kJ of mechanical energy. The temperature rises (TB > TA), showing internal energy increased due to work input.
Example 2: Electrical Work
Adding 1 kJ of energy from an electric heater can also cause the same temperature rise (TB > TA).

This experiment (originally by J.P. Joule) showed that regardless of the method, if the same amount of work is done, the system undergoes the same change in internal energy. Hence, internal energy depends only on the state, not on the path – it is a state function.
Key Equation for an Adiabatic Process: ΔU = wad
Sign convention (IUPAC):
- w > 0: Work done on the system → internal energy increases.
- w < 0: Work done by the system → internal energy decreases.
Familiar State Functions
- Temperature (T)
- Volume (V)
- Pressure (p)
A state function’s change depends only on the initial and final states, not on how the change occurs (the path).
Heat and Internal Energy – Isothermal or Non-Adiabatic Conditions
Now consider a system with thermally conducting walls (e.g., copper container) in contact with a heat reservoir. If water in the system is at temperature TA and the reservoir is at TB, heat flows from the reservoir to the system if TB > TA.
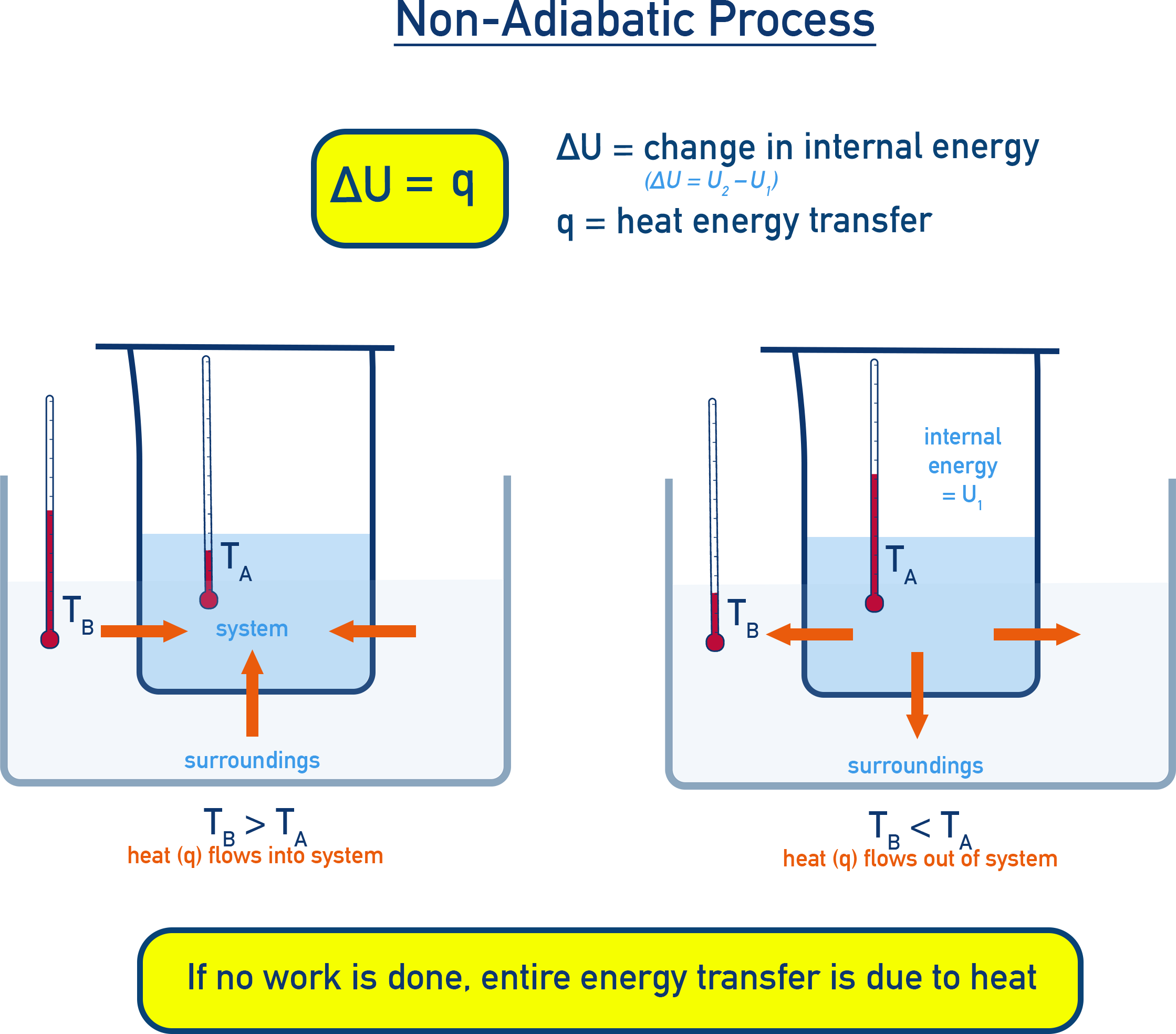
This thermal energy transfer is termed heat (q). If no work is done, the entire energy change is due to heat.
Key Equation at Constant Volume (No Work): ΔU = q
Sign convention (IUPAC):
- q > 0: Heat added to the system → internal energy increases.
- q < 0: Heat lost by the system → internal energy decreases.
General Case: First Law of Thermodynamics
In most real situations, both heat and work affect the internal energy.
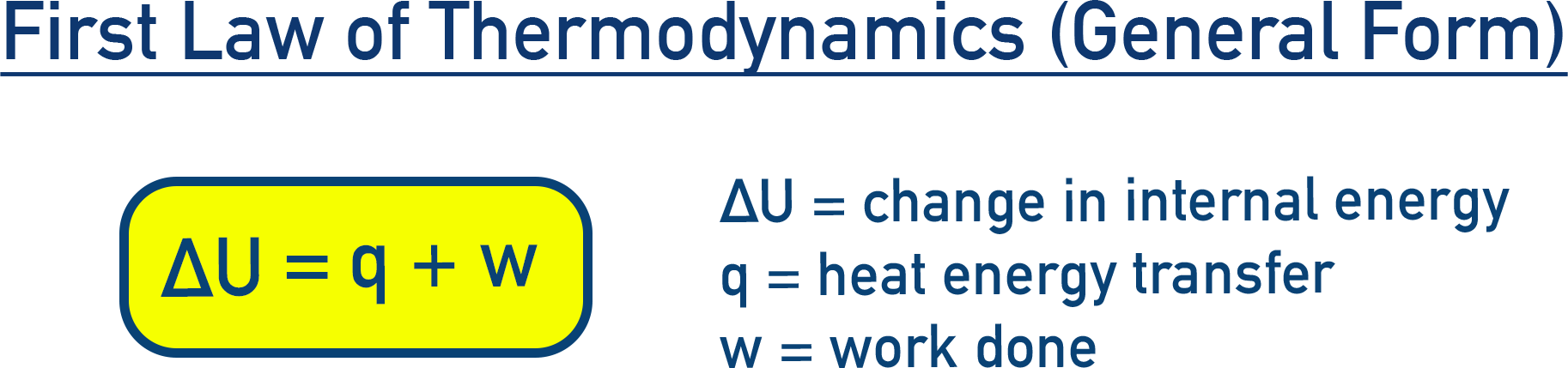
First Law of Thermodynamics (General Form): ΔU = q + w
- ΔU: Change in internal energy
- q: Heat transferred
- w: Work done
Although q and w depend on the path, their sum (ΔU) depends only on the state, reaffirming that internal energy is a state function.
If a system is isolated, i.e., no heat or work transfer then:
q = 0 and w = 0 → ΔU = 0
Important Distinction: Internal energy is not directly measurable in absolute terms. Only changes in internal energy (ΔU) are physically meaningful, unlike properties like volume, which can be directly measured.
Summary
- A system is the part studied and surroundings are everything else.
- Open systems exchange matter and energy, closed systems exchange energy only, isolated systems exchange neither.
- State is described by P, V, T, and composition.
- Work and heat are path dependent while ΔU is a state function.
- For adiabatic processes ΔU equals wad; at constant volume ΔU equals q; in general ΔU equals q plus w.
