Spontaneity
Quick Notes
- A spontaneous process occurs on its own without external intervention.
- Many spontaneous processes are exothermic, but ΔH < 0 is not the only determining factor in whether a reaction is spontaneous.
- Entropy (S) measures disorder and spontaneous processes generally increase entropy (ΔS > 0) .
- Gibbs Free Energy (G) combines ΔH and ΔS:
ΔG = ΔH − TΔS - Criteria for spontaneity:
- ΔG < 0 → spontaneous
- ΔG > 0 → non-spontaneous
- ΔG = 0 → equilibrium
- Second Law: Total entropy of the universe increases in spontaneous processes.
- Third Law: Entropy of a perfect crystal at 0 K is zero.
Full Notes
Introduction
Some processes occur naturally and spontaneously – like the melting of ice at room temperature or rusting of iron – while others require continuous external effort (e.g., compressing a gas).
A spontaneous process is an irreversible process that may only be reversed by an external overall input of energy or work.
Thermodynamics helps us understand and predict which changes are likely to occur on their own. Initially, it was thought that energy release (ΔH < 0) was the only criteria for spontaneity. However, this idea was challenged by spontaneous endothermic processes. The true picture includes entropy and is formalized through the concept of Gibbs energy.
Is Decrease in Enthalpy a Criterion for Spontaneity?
While most spontaneous reactions are exothermic (ΔH < 0), not all are.
Examples: Exothermic and endothermic yet spontaneous
- Combustion of fuels CH4(g) + 2O2(g) → CO2(g) + 2H2O(l); ΔH < 0
- Freezing of water
- Melting of ice at room temperature
- Evaporation of water
- Dissolution of NaCl in water
Hence, enthalpy change alone does not determine spontaneity.
Entropy and Spontaneity
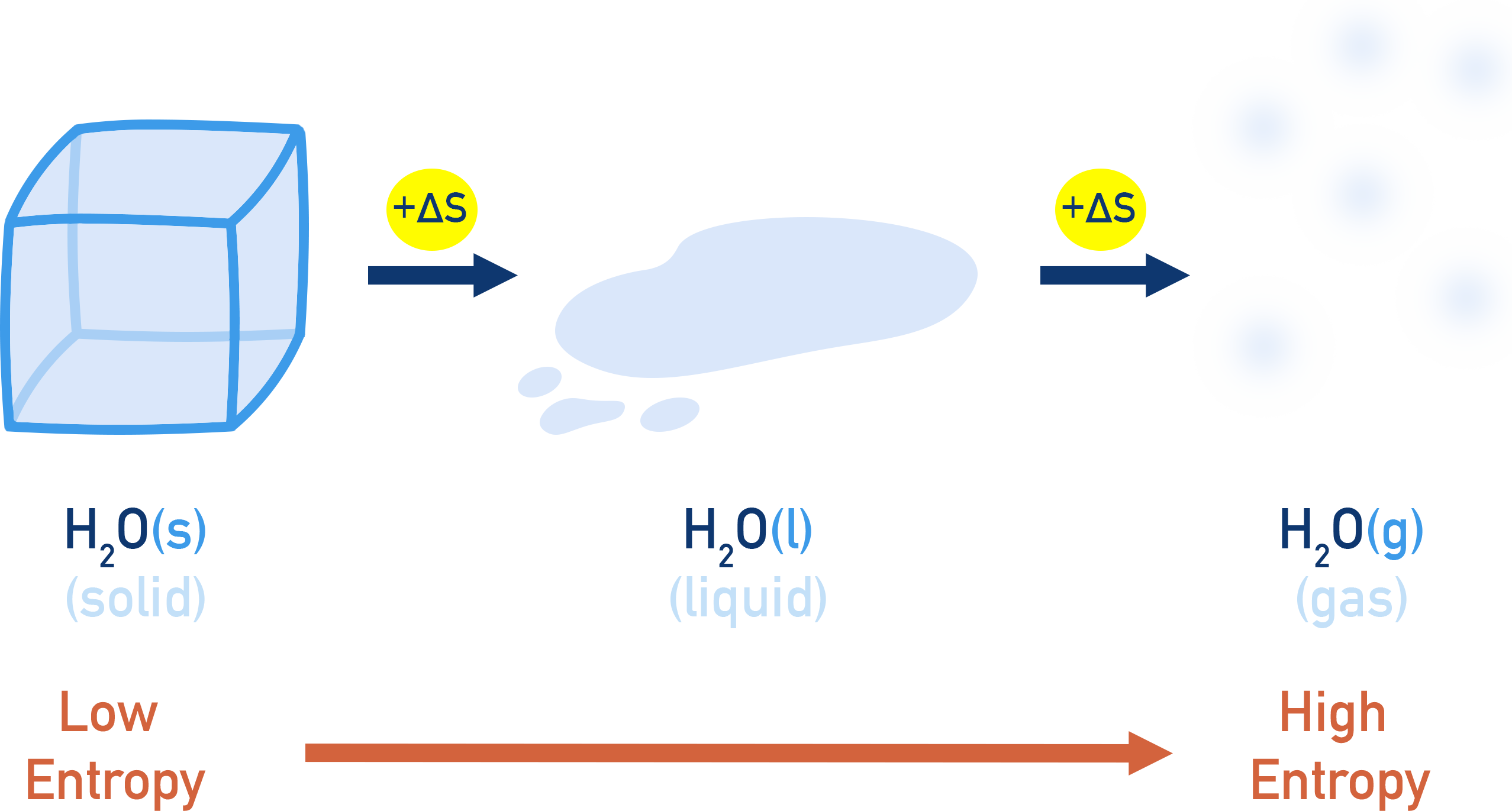
Entropy (S) is a thermodynamic quantity that measures the degree of randomness or disorder in a system. Greater disorder = higher entropy.
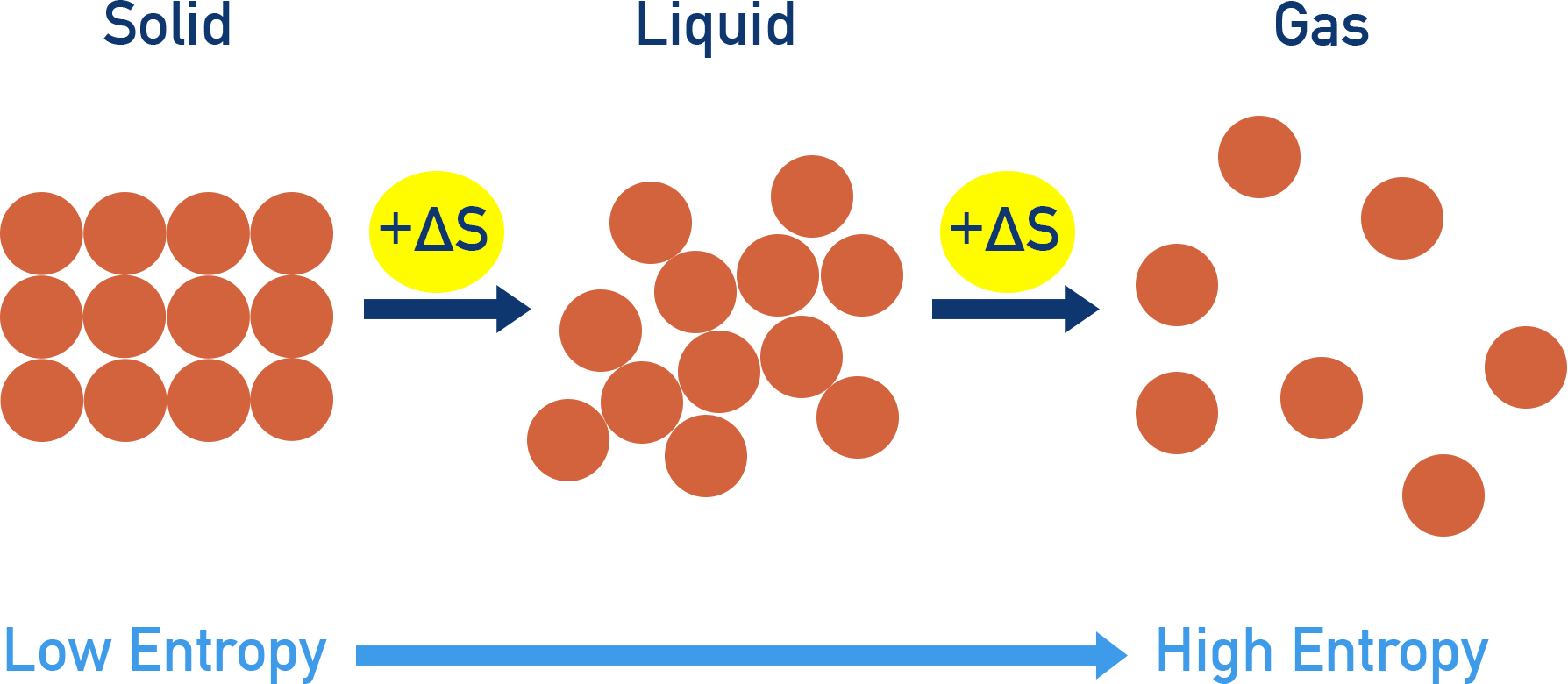
Solid → Liquid → Gas transitions increase entropy.
Dissolving solids or gases in liquids increases entropy.

In a spontaneous process, the entropy of the universe increases.
At equilibrium, entropy becomes maximum and does not change further: ΔStotal = 0
Quantifying Entropy Change (ΔS)
Entropy is a state function (depends only on initial and final states).
Effect of Heat (q) on Entropy
Adding heat increases molecular motion, increasing disorder and causing entropy to increase.
But the same heat added at low temperature causes more disorder than at high temperature.
This is because molecules at lower temperature are more ordered, so heat has more impact.
Therefore, entropy change is inversely proportional to temperature.
Entropy and Reversible Processes
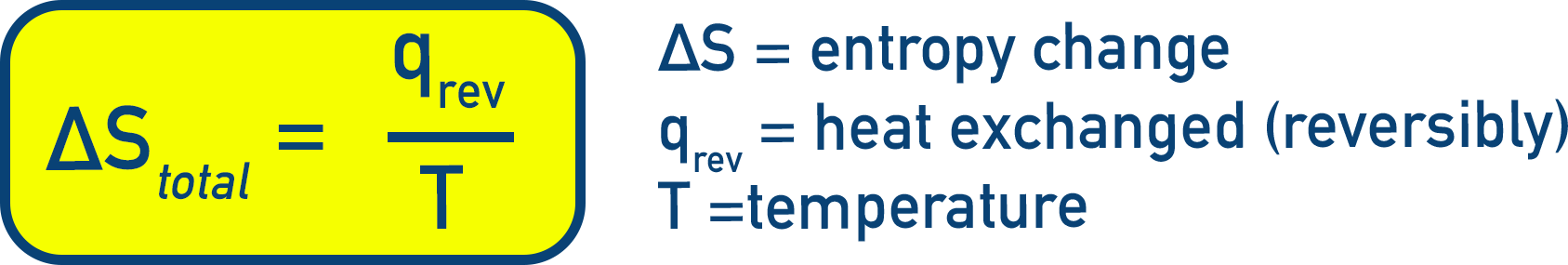
(Equation 5.18)
- ΔS is the entropy change of the system
- qrev is the heat exchanged reversibly
- T is the temperature in Kelvin
This relation holds for all reversible processes.
Discrimination Between Reversible and Irreversible Processes
Let’s consider the isothermal expansion of an ideal gas:
For both reversible and irreversible isothermal expansions: ΔU = 0
However:
- In a reversible expansion:
ΔStotal = ΔSsystem + ΔSsurroundings = 0 - In an irreversible expansion:
ΔStotal > 0
Hence, internal energy (ΔU) cannot allow us to differentiate between reversible and irreversible changes, but entropy (ΔS) can.
This confirms that entropy change — not just energy — is critical to understanding process directionality and irreversibility.
Gibbs Energy and Spontaneity
From the above, we can see that a positive ΔStotal tells us whether a reaction is spontaneous. However, most reactions and processes don’t happen in an isolated system, meaning we need to find another way of determining whether a reaction is spontaneous, based on both ΔHsystem and ΔSsystem.
To combine both ΔH and ΔS into a single predictive function, we use Gibbs free energy (G):
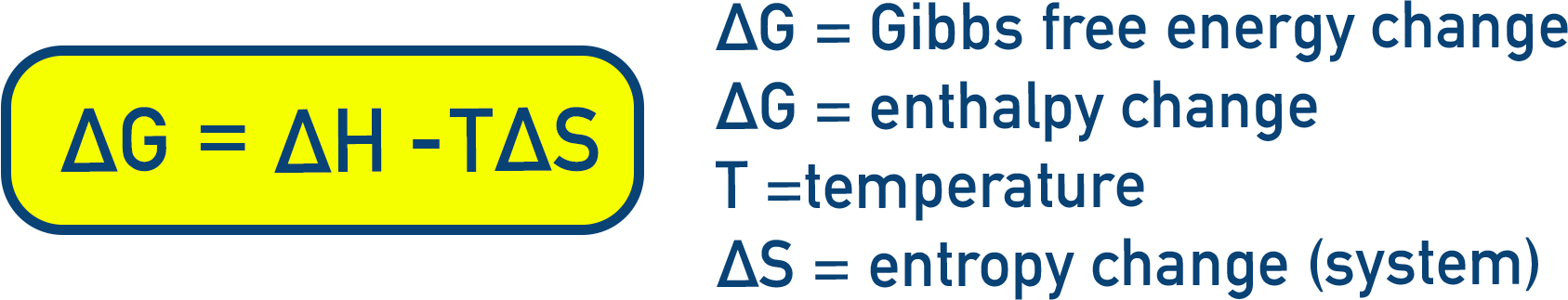
(Equation 5.21)
If:
- ΔG < 0 → spontaneous
- ΔG > 0 → non-spontaneous
- ΔG = 0 → system at equilibrium
Example: Melting of ice at 273 K
ΔH = +6.0 kJ mol⁻¹
ΔS = +22 J mol⁻¹ K⁻¹
At T = 273 K: ΔG = 6000 − (273 × 22) = −6 J mol⁻¹ → Spontaneous
Thus, Gibbs energy provides a clear and quantitative way of determining spontaneity.
Entropy and the Second Law of Thermodynamics
The Second Law of Thermodynamics states:
“In any spontaneous process, the total entropy of the universe increases.”

The law applies even when the system entropy decreases – as long as the surroundings compensate by increasing entropy more.
Equilibrium occurs when: ΔSsystem = −ΔSsurroundings
So that: ΔSuniverse = 0
This law provides a directional arrow to natural processes (i.e. tells us the direction processes will occur in).
Absolute Entropy and the Third Law of Thermodynamics
The Third Law of Thermodynamics states:
“The entropy of a perfectly crystalline substance is zero at absolute zero (0 K).”
This provides a reference point for calculating absolute entropies of substances at any temperature above 0 K.
Mathematically, the entropy change from 0 K to T can be calculated using: ΔS = ∫ (dqrev / T) from 0 to T
Summary
- Spontaneous processes occur without external help and are irreversible.
- Decrease in enthalpy alone does not guarantee spontaneity.
- Entropy measures disorder and increases for spontaneous change.
- Gibbs free energy combines enthalpy and entropy to predict direction.
- The Second Law states total entropy increases for spontaneous change.
- The Third Law gives zero entropy for a perfect crystal at 0 K.
