Alkanes
Quick Notes — Alkanes (CnH2n+2)
- Definition: Saturated hydrocarbons containing only σ-bonds (C–C, C–H); low reactivity, non-polar.
- General formula (acyclic): CnH2n+2; nomenclature: IUPAC “-ane”, straight & branched chains.
- Isomerism: Chain isomerism begins at C4 (e.g., n-butane vs isobutane).
- Physical trends: boiling point increases with molar mass.
- Preparations:
- Hydrogenation of alkenes/alkynes (Ni/Pt/Pd).
- From alkyl halides: reduction (Zn/HCl) and Wurtz coupling (2 R–X + 2 Na → R–R).
- From carboxylates: Decarboxylation (soda-lime) and Kolbe electrolysis (dimerisation).
- Key reactions:
- Free-radical halogenation (Substitution): needs UV light and follows initiation/propagation/termination. Multiple substitutions possible.
- Combustion: CnH2n+2 + (3n+1)/2 O2 → n CO2 + (n+1) H2O (incomplete combustion forms CO and soot).
- Controlled oxidation (catalytic): CH4 → CH3OH (Cu), HCHO (Mo2O3), RCOOH ((CH3COO)2Mn) and 3°-H alkanes form 3° alcohols (KMnO4).
- Isomerisation: straight chain to branched chain using AlCl3/HCl, heat.
- Aromatization: form arenes using Pt or Cr/Mo oxides, ~773 K, 10–20 atm.
- Steam reforming: CH4 + H2O → CO + 3 H2 (Ni, ~1273 K).
- Pyrolysis (cracking): large alkanes broken down into smaller alkanes/alkenes/H2 using Pt/Pd/Ni and a high T.
- Conformations: Free rotation about C–C
staggered (lowest energy) > eclipsed (torsional strain).
Can be represented with Newman & sawhorse projections.
Full Notes
Alkanes are the simplest type of hydrocarbons, consisting of carbon and hydrogen atoms with only single bonds. They are also known as paraffins, indicating their relatively low reactivity. They serve as fuels and starting materials in various chemical industries.
Nomenclature and Isomerism
Nomenclature
The longest continuous carbon chain is identified as the parent chain, and substituents are named as prefixes. The suffix “-ane” denotes saturated hydrocarbons. The numbering of the chain begins from the end nearer to a substituent.
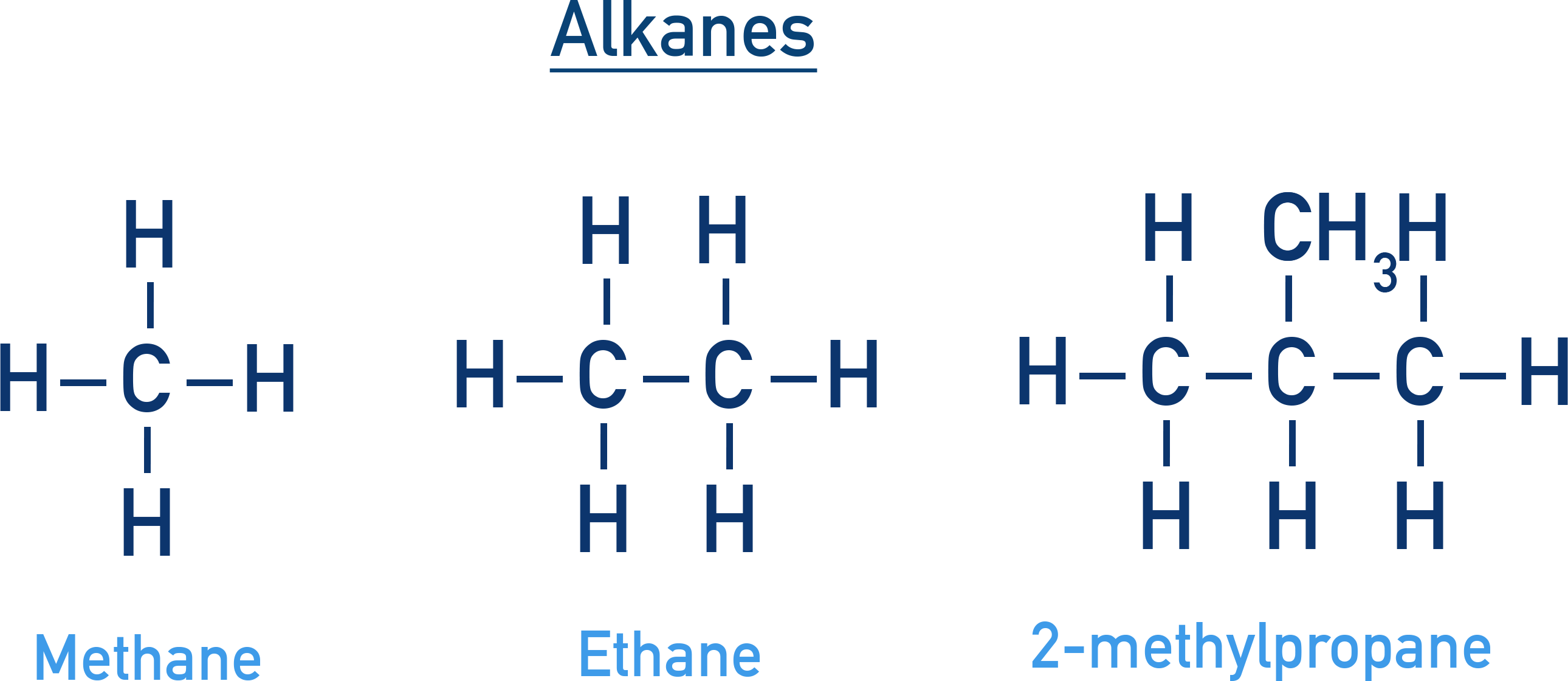
Isomerism
Chain isomerism: Carbon backbones are arranged differently
Begins from butane (C4H10) onwards.
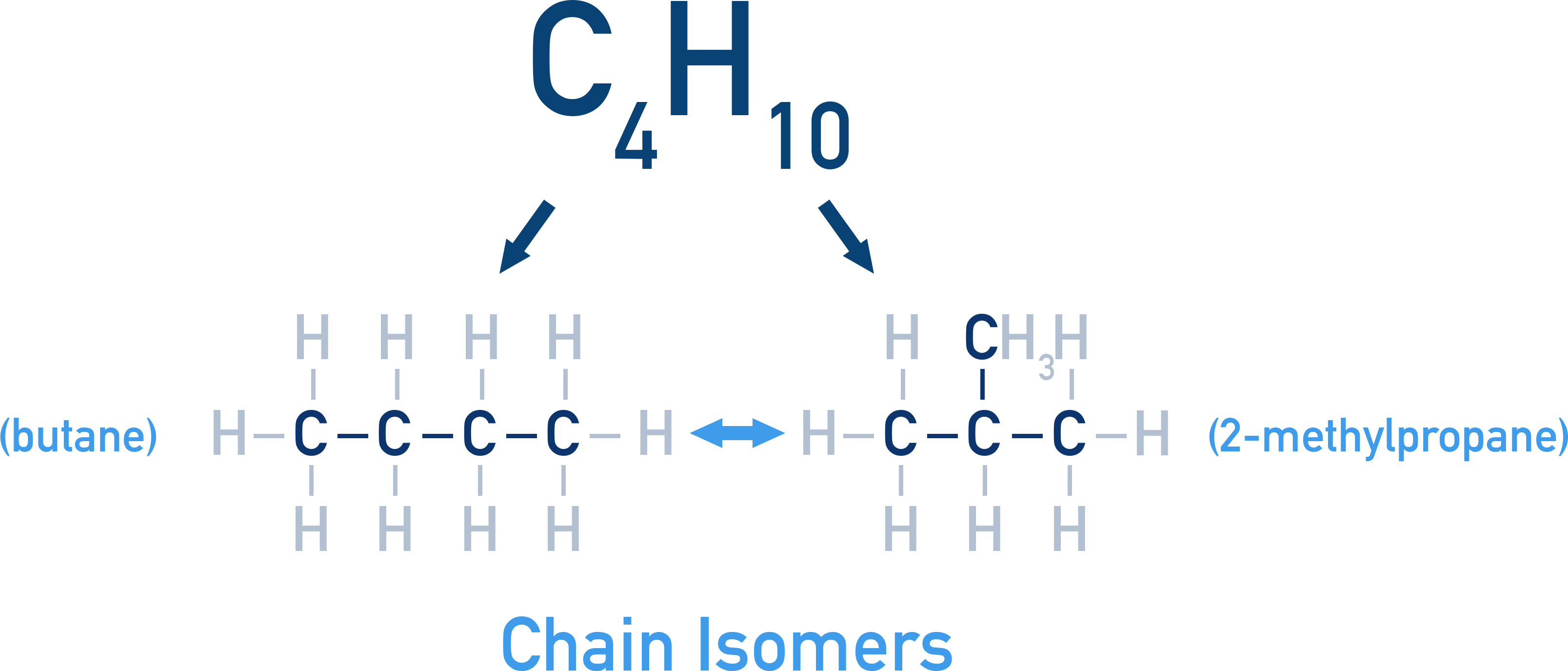
Example: C4H10 – n-butane and isobutane
Preparation of Alkanes
There are several methods for preparing alkanes in the laboratory and industrially. These methods generally involve reduction or elimination reactions starting from compounds like alkenes, alkynes, alkyl halides, or carboxylic acids. Each method provides insight into the synthetic versatility of hydrocarbons.
From Unsaturated Hydrocarbons
Hydrogenation of alkenes or alkynes in the presence of catalysts (Ni, Pt, Pd) leads to the formation of alkanes.
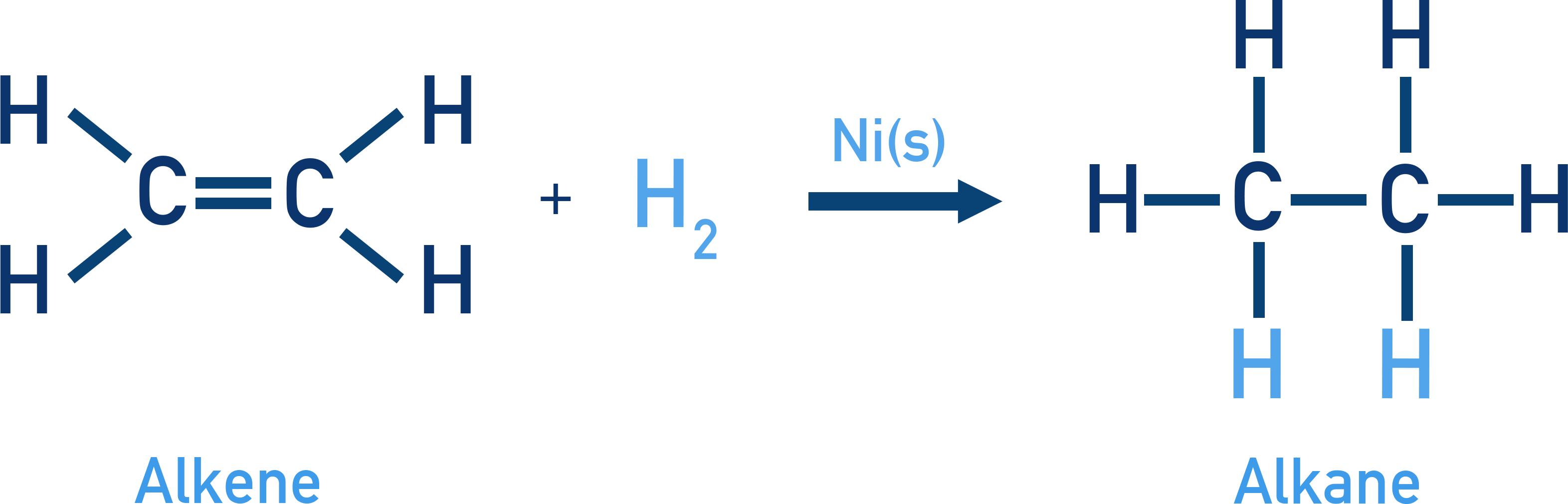
Example CH2=CH2 + H2 → CH3–CH3
From Alkyl Halides
Reduction using Zn/HCl gives alkanes.
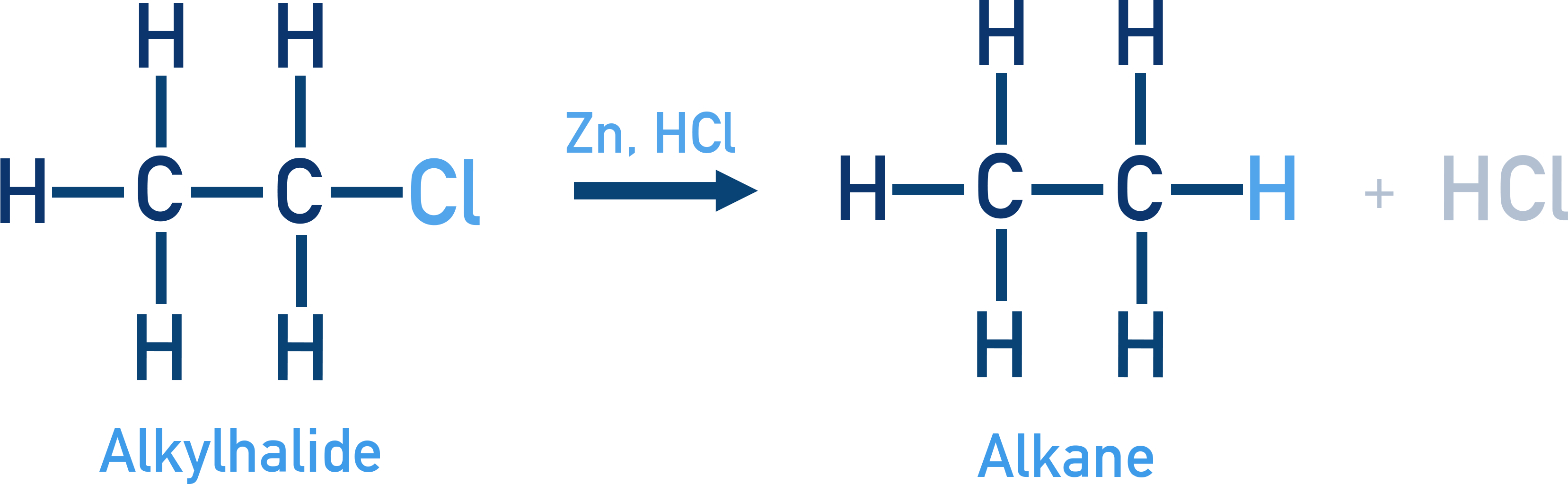
Example CH3CH2Cl + 2[H] → CH3CH3 + HCl
Wurtz reaction is another method used to prepare higher alkanes by reacting alkyl halides with sodium metal in dry ether (a moisture-free solvent).
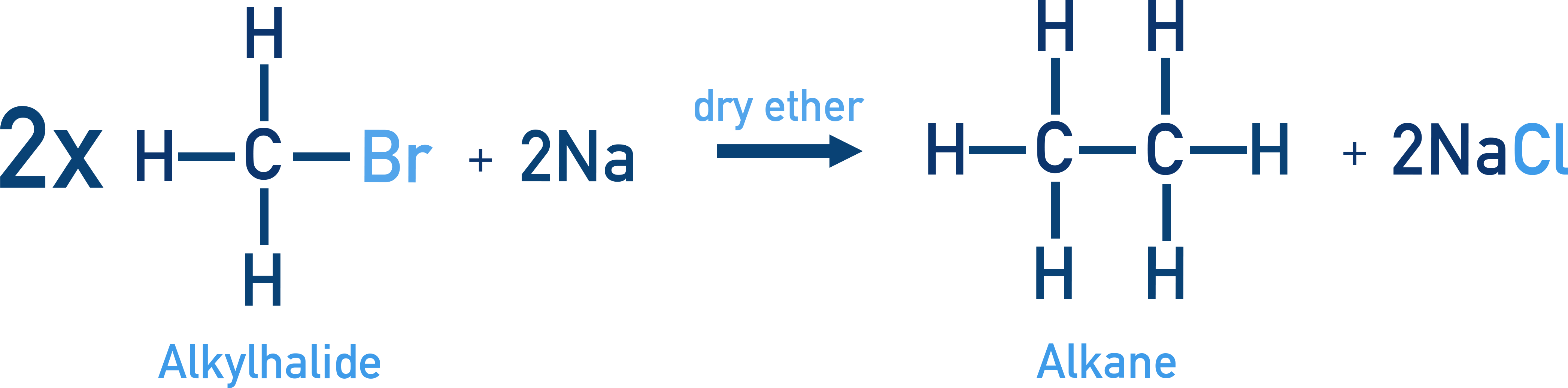
Example CH3Br + 2Na + BrCH3 → CH3–CH3 + 2NaBr
From Carboxylic Acids
Decarboxylation using soda lime:
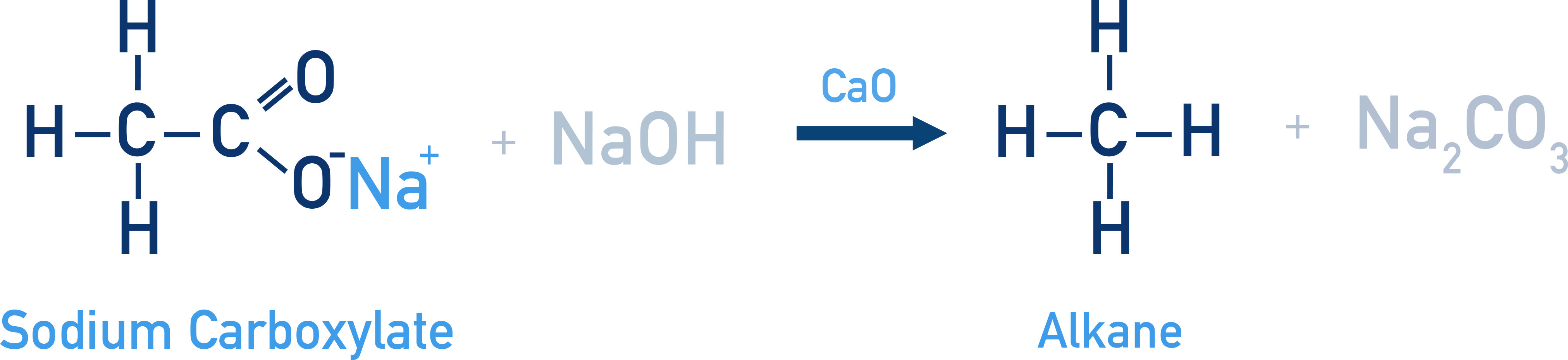
Example CH3COONa + NaOH → CH4 + Na2CO3
Kolbe’s Electrolysis: Dimerization to form alkanes.
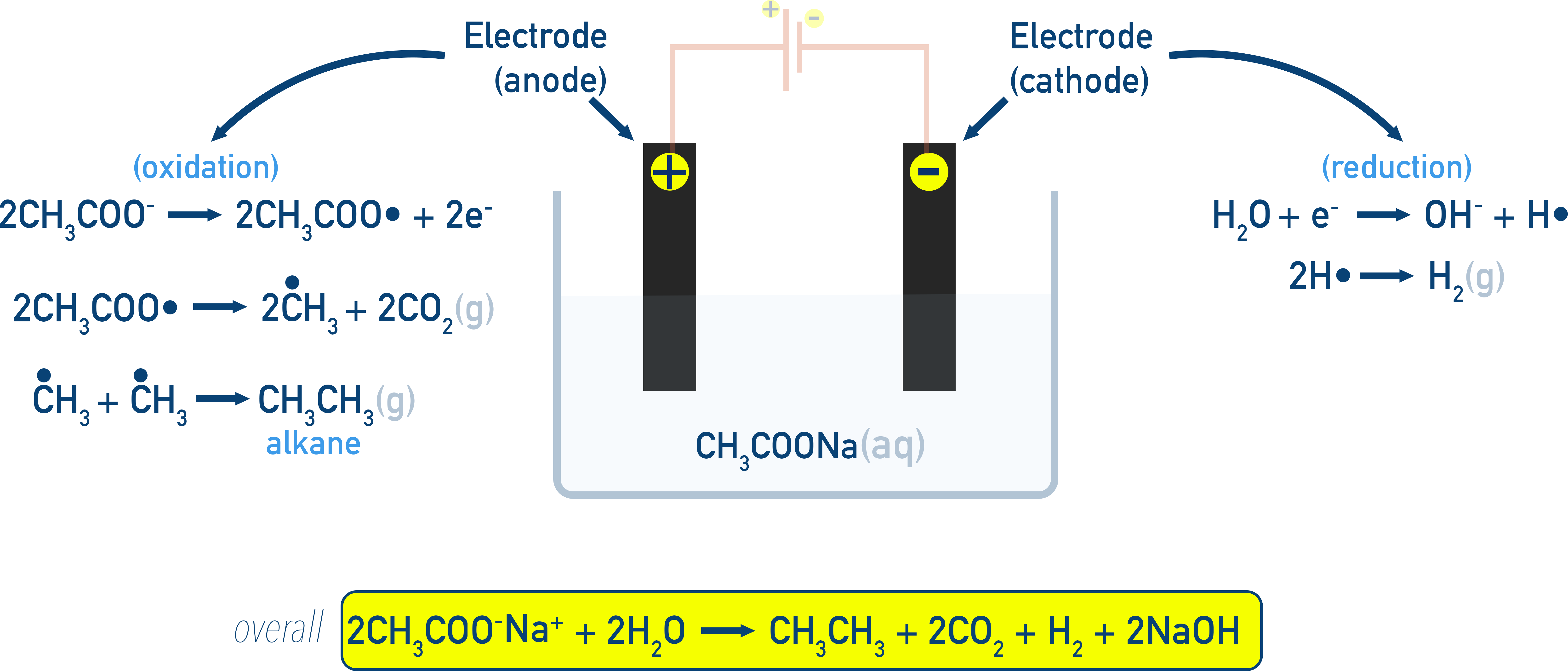
Example 2CH3COONa → C2H6 + 2CO2 + H2 + 2NaOH
Properties of Alkanes
Physical Properties
- Alkanes are non-polar molecules.
- Boiling points increase with molecular weight.
- Insoluble in water but soluble in organic solvents.
- Gaseous (C1–C4), liquid (C5–C17), solid (>C17) at room temperature.
Chemical Properties
The chemical behavior of alkanes is limited due to the strength of the C–C and C–H sigma bonds and the absence of polar functional groups.
However, under specific conditions, they can participate in useful chemical transformations such as halogenation, combustion, and oxidation.
Substitution Reactions – Halogenation
Occurs via free radical mechanism. Alkanes can react with halogens in the presence of ultraviolet (UV) light, producing a mixture of products.
Example Chlorine + methane forming chloromethane
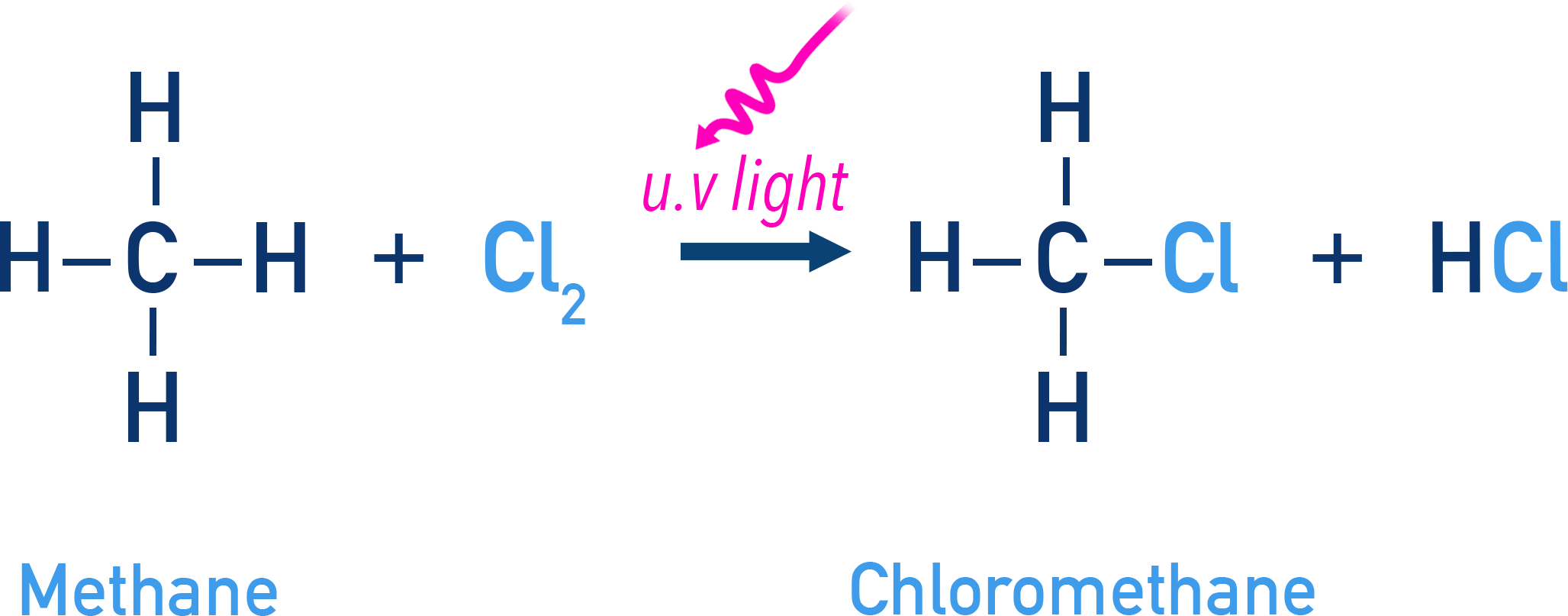
UV light is required to initiate the reaction.
The reaction is an example of free-radical substitution and occurs in several steps. We can show how the reaction occurs using a mechanism.
Free-Radical Substitution Mechanism
Mechanism involves:
- Initiation: Cl2 → 2Cl•
- Propagation: CH4 + Cl• → CH3• + HCl
- Termination: CH3• + Cl• → CH3Cl
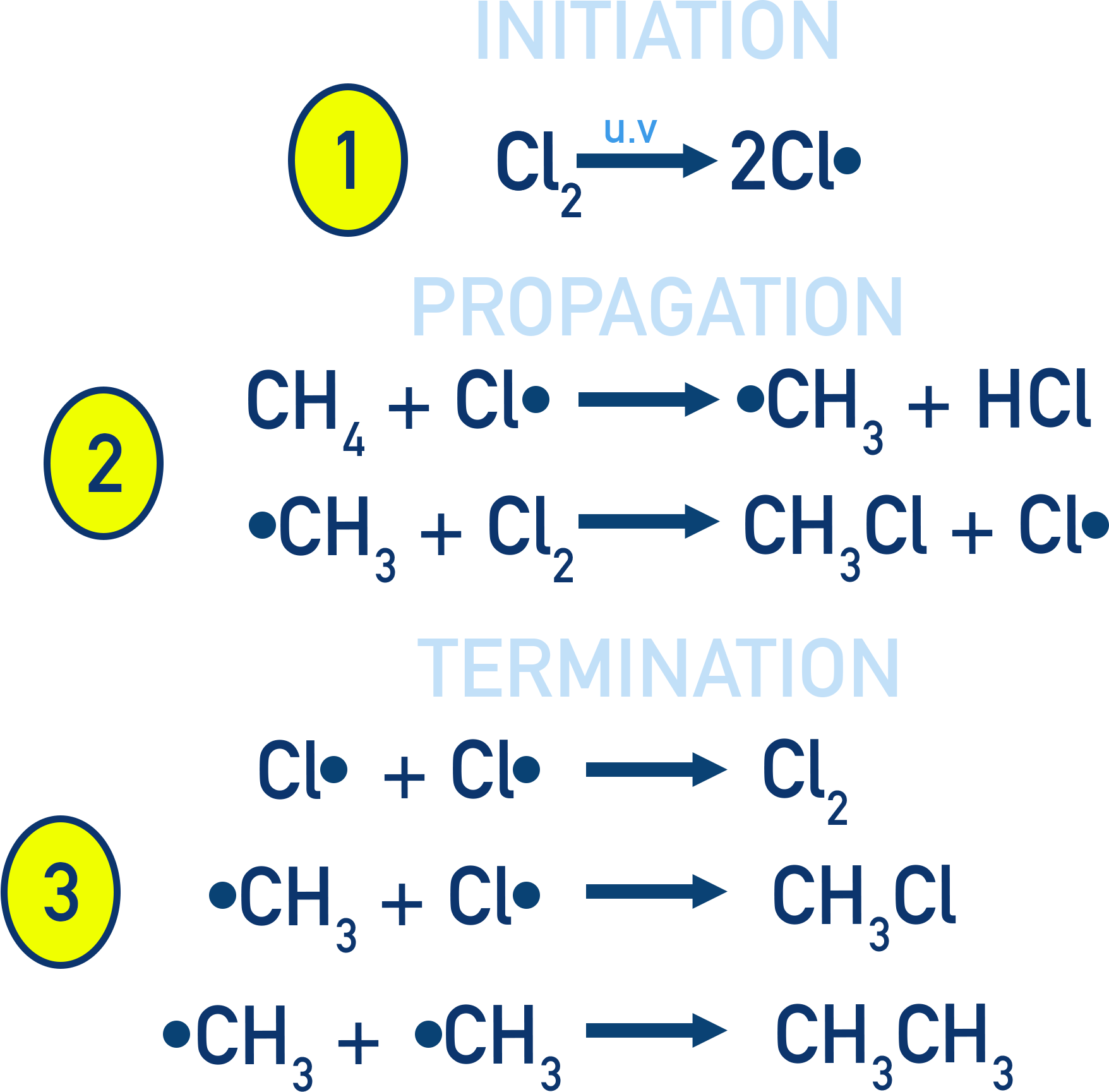
Step 1: Initiation (Radicals Are Formed)
UV light provides energy to break the Cl–Cl bond by homolytic fission. Each chlorine atom ends up with an unpaired electron (•), making it a radical.
Step 2: Propagation (Radicals React and Regenerate)
Radicals react to form new radicals in a chain reaction. Chlorine radical reacts with methane, forming a methyl radical. Methyl radical reacts with Cl2, forming chloromethane and a new Cl• radical. The process continues, leading to further substitutions.
Step 3: Termination (Radicals Are Removed)
Radicals combine to form stable (non-radical) molecules, stopping the reaction. There are several possible termination reactions. Termination stops the chain reaction.
Limitations of Free-Radical Substitution
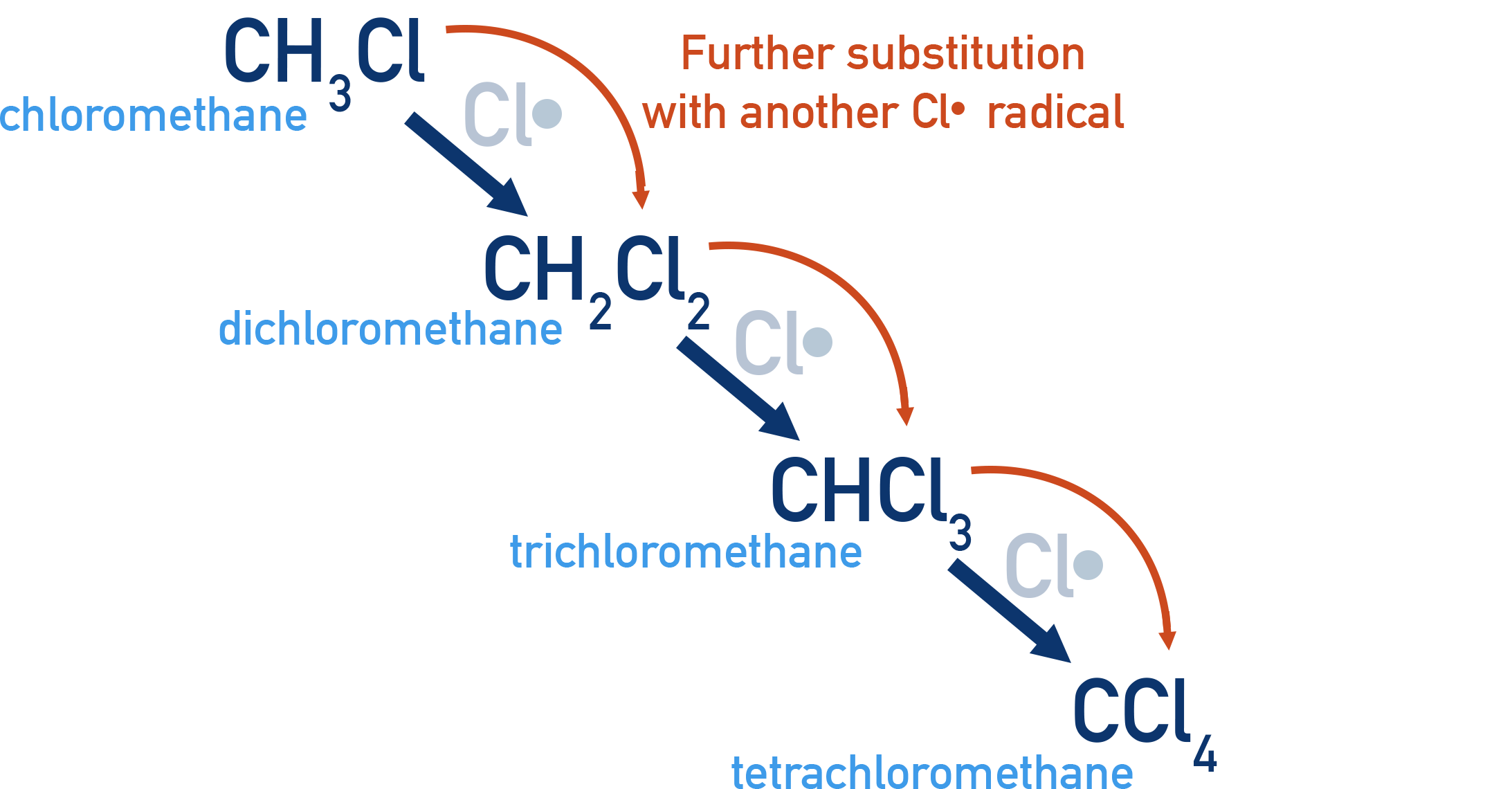
- Further substitution can occur, leading to a mixture of products.
- It is difficult to control, leading to multiple side reactions.
Combustion
Alkanes undergo combustion when heated in the presence of air or dioxygen, producing carbon dioxide (CO2), water (H2O), and a large amount of heat.
Example
Methane (CH4): CH4 + 2O2 → CO2 + 2H2O; ΔH° = −890 kJ mol−1
General Combustion Equation: CnH2n+2 + (3n+1)/2 O2 → nCO2 + (n+1)H2O
Incomplete Combustion: Occurs with limited oxygen. Produces carbon black (soot).
Applications: used in ink, printer ink, black pigments, filters.
Controlled Oxidation
Alkanes, when heated with a regulated supply of dioxygen/air and in the presence of catalysts at high temperature and pressure, can form various oxidation products.
Important Reactions:
Example
Formation of Methanol: 2CH4 + O2 → 2CH3OH
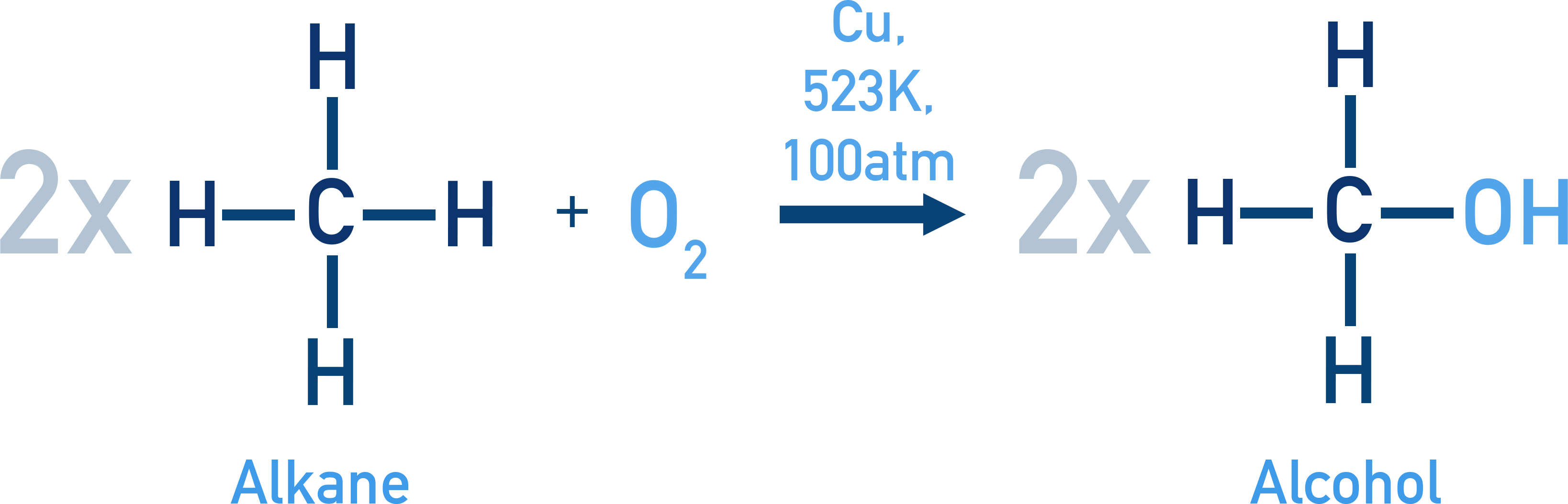
Catalyst: Cu at 523 K and 100 atm (Product: Methanol)
Example Formation of Methanal (Formaldehyde): CH4 + O2 → HCHO + H2O
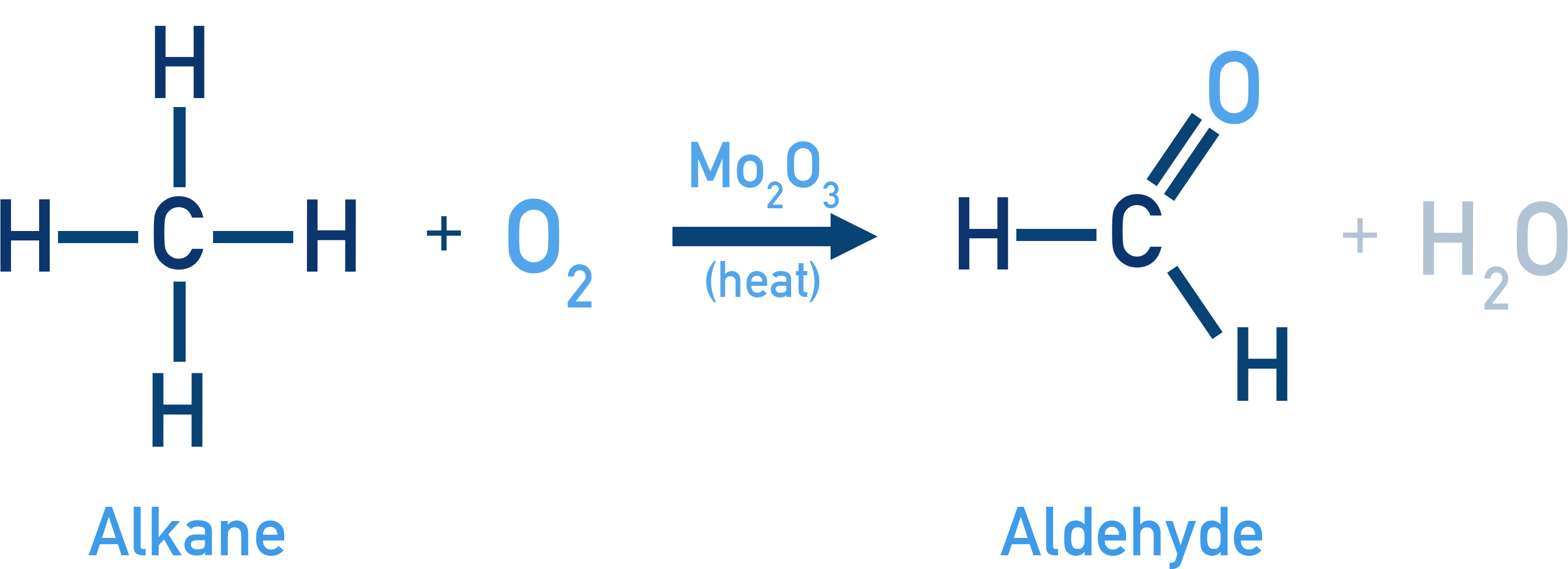
Catalyst: Mo2O3 (heat) (Product: Methanal)
Example Formation of Ethanoic Acid (Acetic Acid): 2CH3CH3 + 3O2 → 2CH3COOH + 2H2O
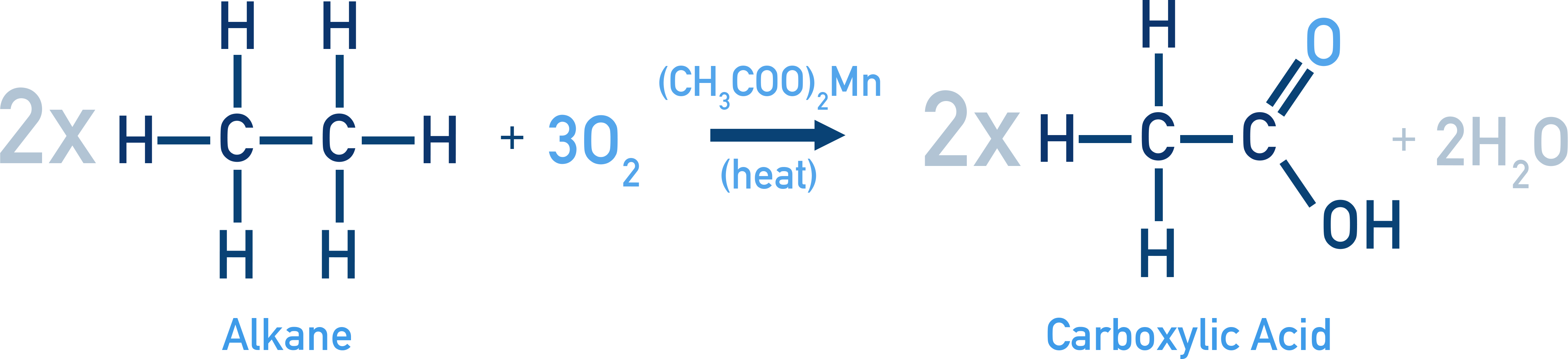
Catalyst: (CH3COO)2Mn (heat) (Product: Ethanoic acid)
Oxidation of Tertiary Alkanes to Alcohols:
Alkanes with tertiary H can be oxidized to tertiary alcohols by KMnO4.
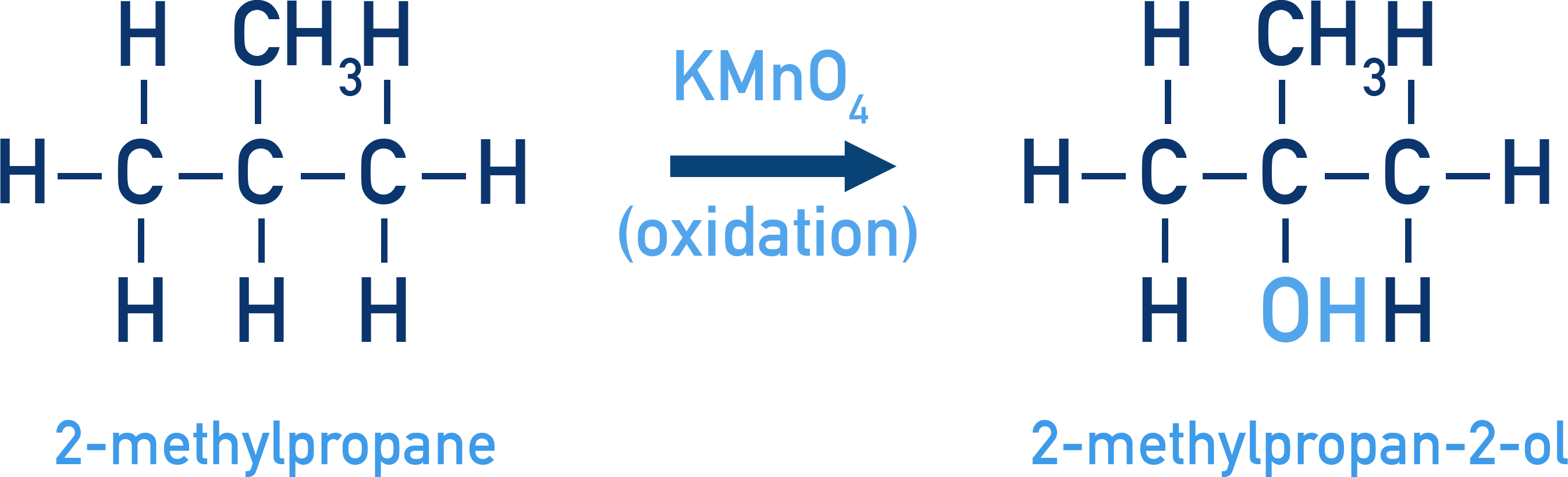
Example (CH3)3CH + [O] → (CH3)3COH
(2-Methylpropane → 2-Methylpropan-2-ol)
Note: Ordinary alkanes are resistant to oxidation. Selective oxidation requires proper conditions and catalysts.
Isomerisation
Heating with anhydrous AlCl3/HCl rearranges straight-chain to branched alkanes.
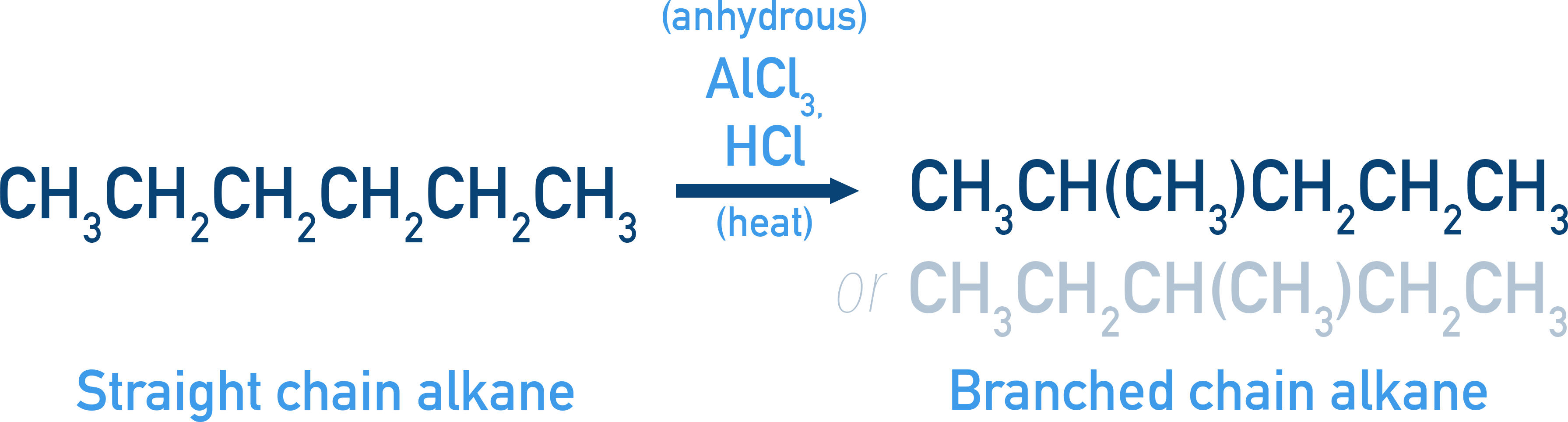
Example hexane → 2-Methylpentane or 3-methylpentane
Aromatization
Aromatization is a chemical reaction in which n-alkanes (straight-chain alkanes) having six or more carbon atoms are converted into aromatic compounds such as benzene.
Conditions Required:
- Temperature: 773 K
- Pressure: 10–20 atm
- Catalyst: Oxides of vanadium (V2O5), molybdenum (Mo2O3), or chromium (Cr2O3) supported on alumina
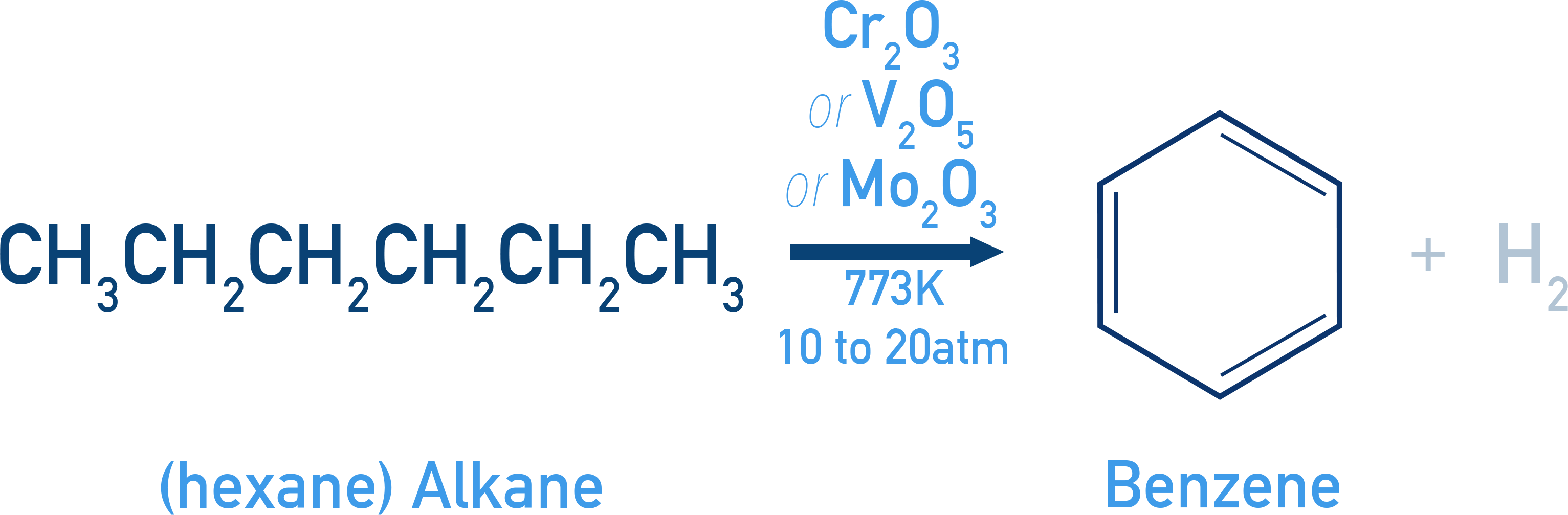
Example n-Hexane → Benzene + H2 (Pt/773 K, 10–20 atm)
Reaction with Steam
Methane reacts with steam (water vapor) at high temperature in the presence of a nickel catalyst to produce carbon monoxide (CO) and dihydrogen gas (H2).
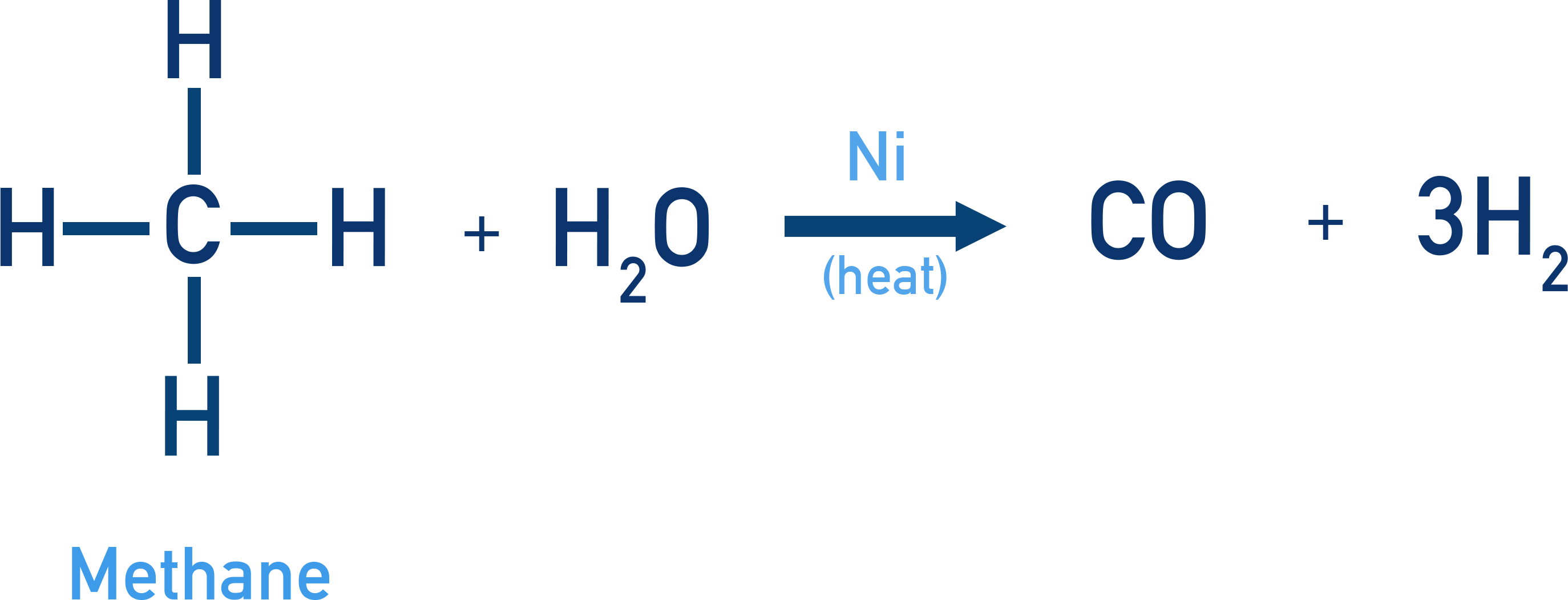
Reaction CH4 + H2O → CO + 3H2 (Conditions: Ni catalyst, 1273 K)
Key Points: This is an important industrial method for the production of dihydrogen gas. The reaction occurs at a very high temperature (1273 K).
Pyrolysis (Cracking)
Pyrolysis (or cracking) is the decomposition of higher alkanes into smaller hydrocarbons (alkanes, alkenes, hydrogen) by heating to a high temperature, typically in the absence of air.
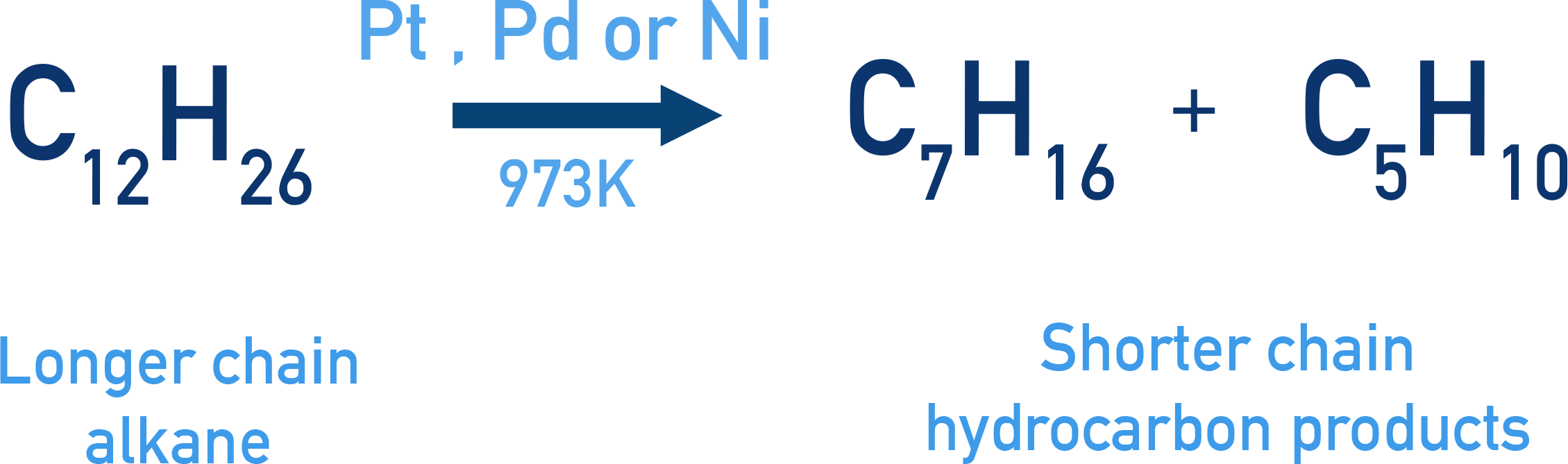
Example C12H26 → C7H16 + C5H10 + other products (Catalyst: Pt/Pd/Ni, Temperature: 973 K)
Dodecane (from kerosene oil) cracks to give useful fuel components like heptane and pentene. Catalysts (Pt, Pd, Ni) are used to facilitate the reaction.
Conformations of Alkanes
Alkanes contain C–C sigma (σ) bonds, which allow free rotation around the bond axis. This rotation gives rise to different spatial arrangements of atoms called conformations or conformers (also known as rotamers).
Conformations of Ethane (C2H6)
Ethane has a C–C single bond; each carbon is attached to three hydrogen atoms. Rotation around the C–C bond changes the relative positions of H-atoms, producing different conformations. These are called conformational isomers.
Types of Conformations
- Eclipsed Conformation: H-atoms on the front and back carbon are aligned. This is the least stable conformation due to maximum repulsion (torsional strain).
- Staggered Conformation: H-atoms are positioned as far apart as possible. This is the most stable conformation.
- Skew Conformations: All intermediate arrangements between eclipsed and staggered.
Note: Bond angles and bond lengths remain the same in all conformations.
Torsional Strain
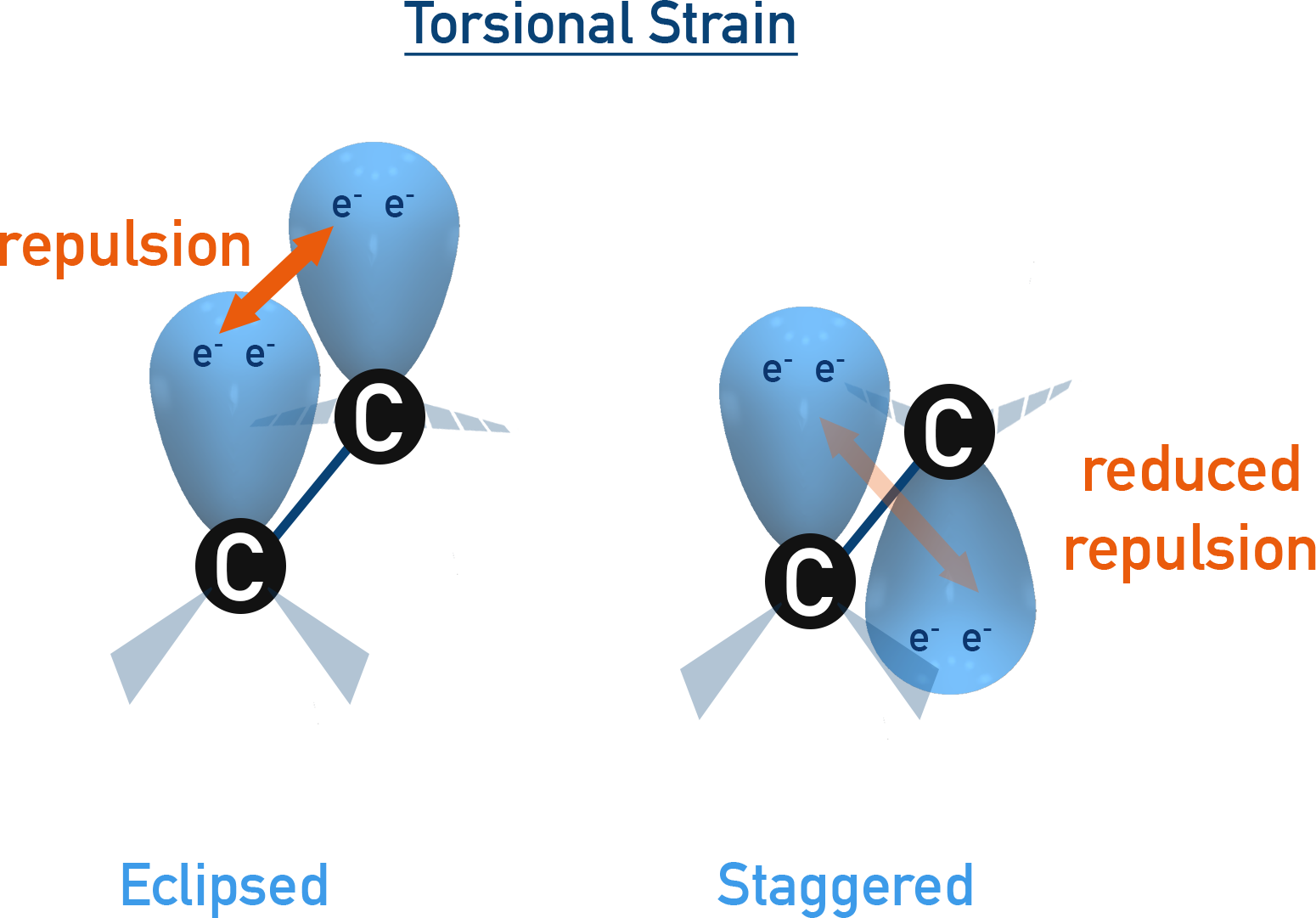
In eclipsed conformation, the bonding electron pairs on adjacent carbon atoms are aligned, leading to maximum repulsion. This causes torsional strain, raising the energy of the molecule.
In staggered conformation, the electron pairs are as far apart as possible, leading to minimum repulsion and greater stability. Hence, staggered is the most stable, while eclipsed is the least stable form.
Projections to Represent Conformations
1. Sawhorse Projection: Molecule is viewed along the molecular axis.
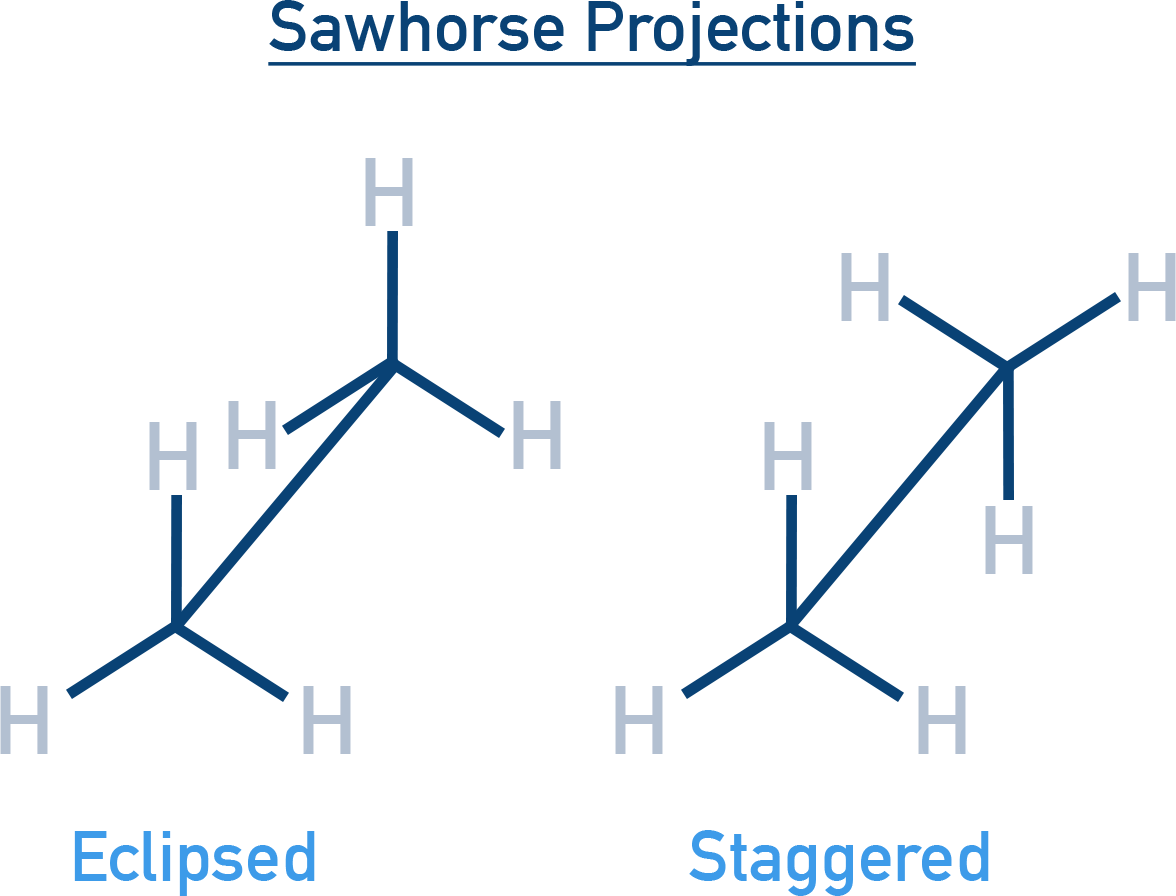
The front carbon is shown lower, rear carbon higher. Shows C–C bond as a diagonal line, with H-atoms at 120° angles. Helps visualize relative positions of atoms on both carbons.
2. Newman Projection: Molecule is viewed head-on along the C–C bond.
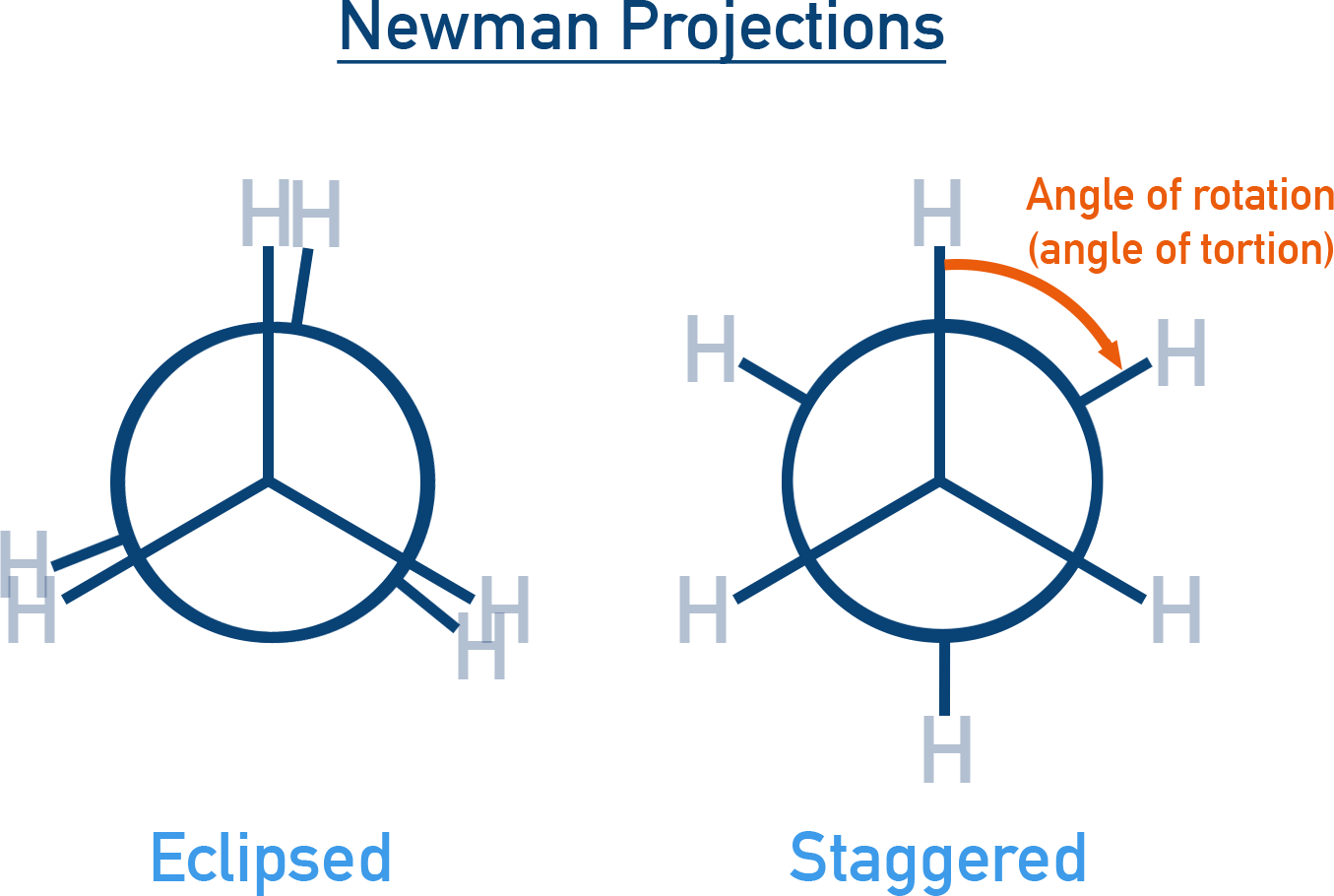
Front carbon is a dot, rear carbon is a circle. H-atoms are shown at 120° angles. Clearly shows angle of rotation (dihedral angle).
Summary
- Alkanes are saturated hydrocarbons with general formula CnH2n+2.
- IUPAC naming uses the longest chain and substituent locants.
- Chain isomerism begins at C4 and increases with carbon number.
- Key preparations include hydrogenation, reductions of halides, decarboxylation, and Kolbe electrolysis.
- Reactivity features free-radical halogenation, combustion, controlled oxidation, and skeletal rearrangements.
- Aromatization, steam reforming, and cracking are important industrial processes.
- Conformations of alkanes arise from rotation about C–C σ-bonds with staggered being most stable.
