Fundamental Concepts in Organic Reaction Mechanism
Quick Notes
- Bond Fission
- Homolytic: Bond splits evenly and forms free radicals.
- Heterolytic: Bond splits unevenly and electrons go to one atom, forming a carbocation and carbanion.
- Stability of Carbocations
- Order: tertiary > secondary > primary > methyl
- Stabilized by positive inductive (+I) effect and hyperconjugation.
- Substrate & Reagent
- Substrate = reactant organic molecule.
- Reagents = attacking species (nucleophile/electrophile).
- Types of Electron Movement
- Curved arrows show direction of electron pair movement.
- Nucleophile: donates electrons
- Electrophile: accepts electrons
- Inductive Effect (I)
- Electron shift via σ-bond due to electronegativity.
- Positive inductive effect (+I): electron releasing (e.g., alkyl)
- Negative inductive effect (–I): electron withdrawing (e.g., NO2, CN)
- Resonance occurs due to delocalization of π-electrons
- Resonating structures = real hybrid.
- Affects bond length, stability.
- Resonance Effect (R)
- Positive resonance effect (+R): Groups donate e− via π bond (e.g., OH, NH2)
- Negative resonance effect (–R): Groups withdraw e− via π bond (e.g., NO2)
- Electromeric Effect (E) is the temporary shift of π electrons towards electrophile.
- Hyperconjugation
- Delocalization of σ-electrons of C–H bond adjacent to π-system or empty p-orbital.
- Explains stability of carbocations & alkenes.
Full Notes
Fission of a Covalent Bond
In organic chemistry, understanding how bonds break (fission) is crucial to following reaction mechanisms.
Fission of a Covalent Bond
A covalent bond can break in two ways:
Homolytic cleavage:
Bond breaks evenly and each atom gets one electron.

Forms Free radicals – neutral species with an unpaired electron that are highly reactive.
Heterolytic cleavage:
Bond breaks unevenly and both shared electrons go to one atom.
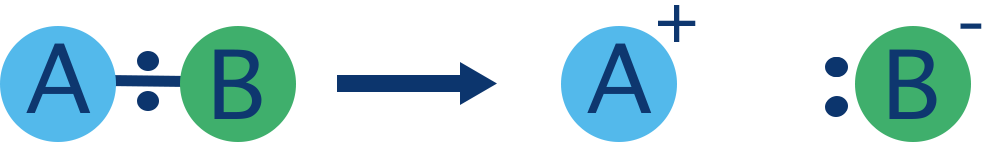
Can cause carbocations and carbanions to form:
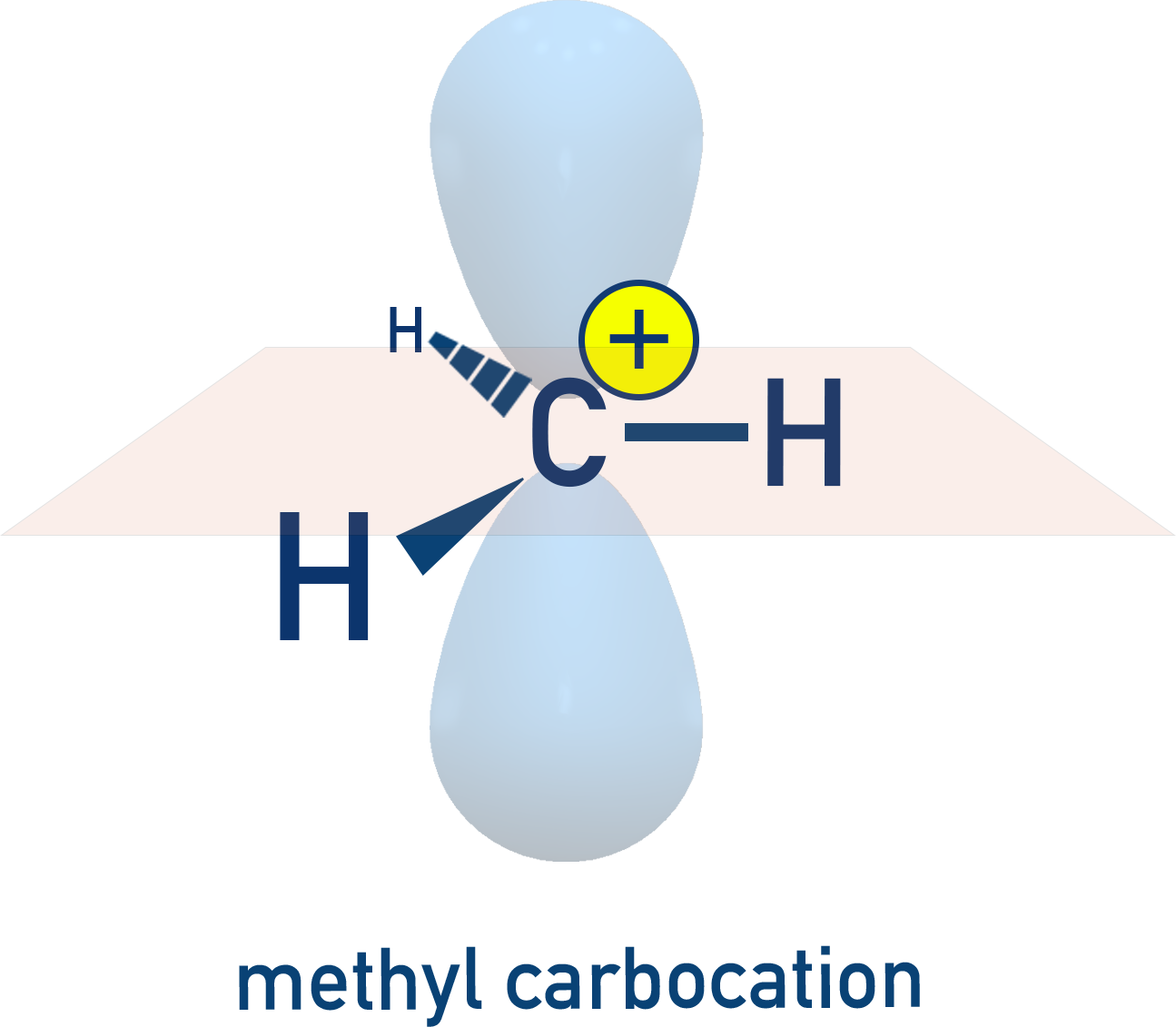
Carbocations (⁺) – electron-deficient, unstable, sp² hybridised (e.g. methylcarbocation, CH₃⁺).
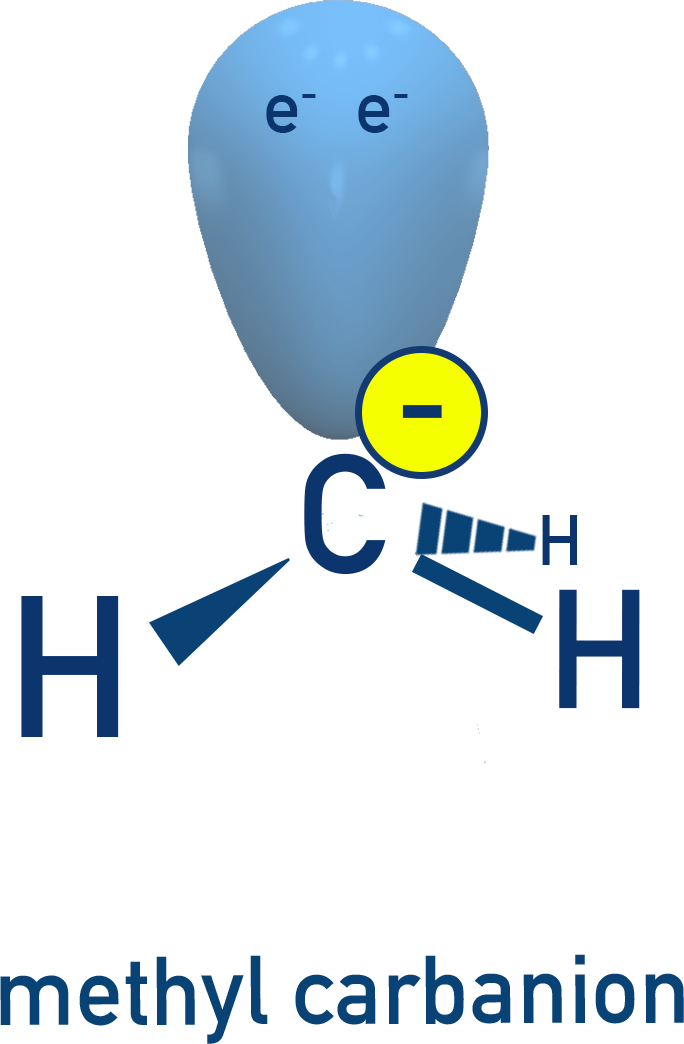
Carbanions (⁻) – electron-rich, also unstable, sp³ hybridised (e.g. methylcarbanion, CH₃⁻).
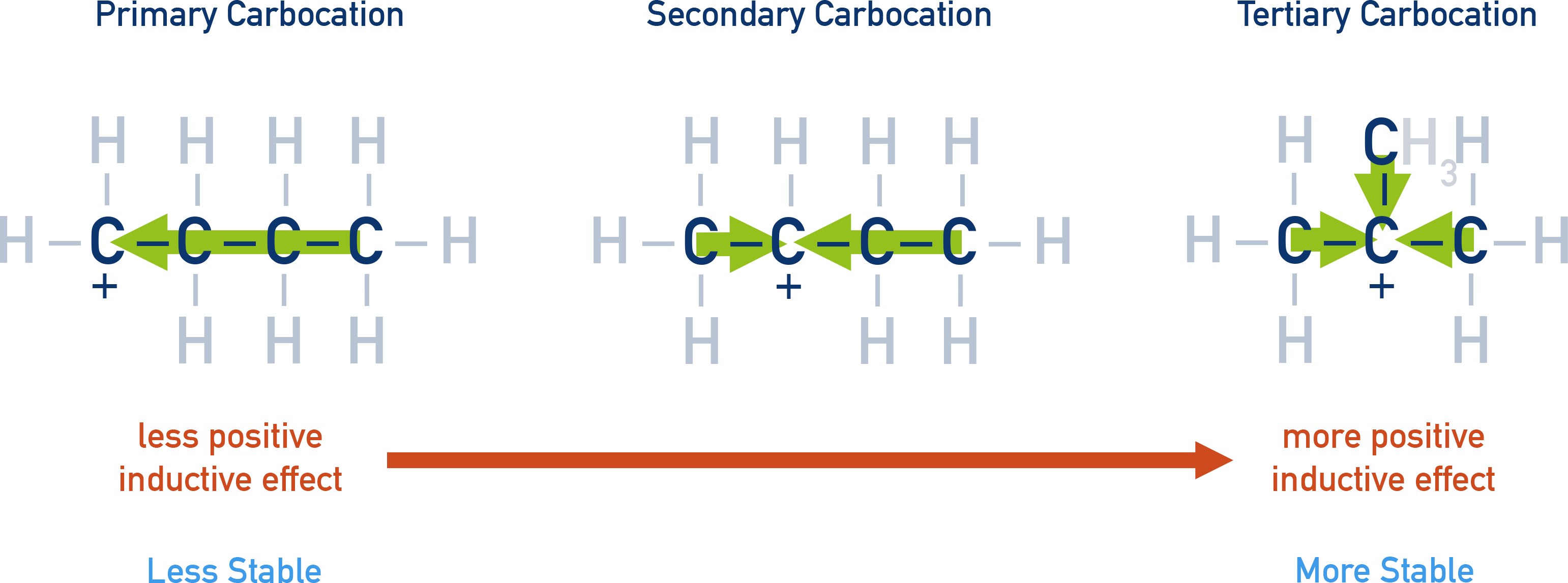
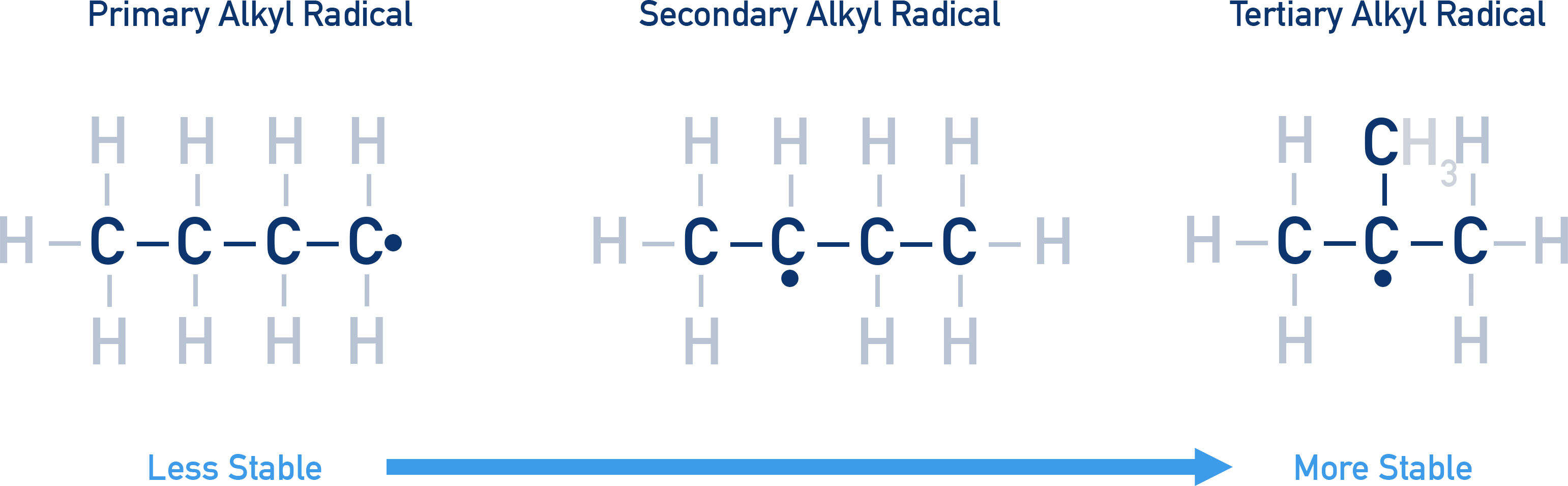
Stability order:
Carbocation stability order: Primary < Secondary < Tertiary
Free radicals: CH₃• < CH₃CH₂• < (CH₃)₂CH• < (CH₃)₃C•
Substrate and Reagent
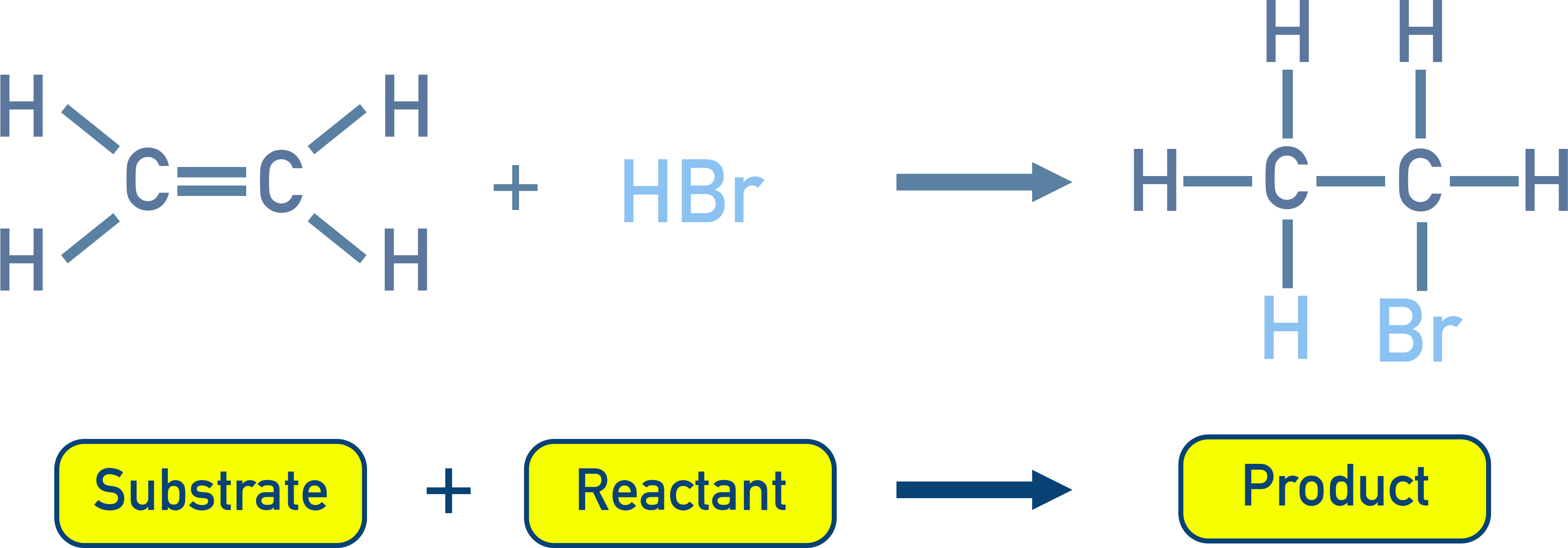
Substrate:
The molecule that provides the carbon atom for new bond formation.
Reagent:
The attacking species (e.g. Br₂, HCl).
Electrophiles (E⁺):
Electron-deficient, attack electron-rich sites (e.g. CH₃⁺, NO₂⁺).
Nucleophiles (Nu⁻):
Electron-rich, donate electron pairs to electrophiles (e.g. OH⁻, CN⁻).
In polar reactions, nucleophiles attack electrophilic centres.
Electron Movement in Organic Reactions
We can represent how electrons move during bond breaking and making using curved or 'curly' arrows:
- Double-headed arrow: movement of an electron pair.
- Single-headed arrow: movement of a single electron (in free radical reactions).
Curved arrows help represent bond formation, cleavage, and resonance.
Used in reaction mechanisms to track electron flow from nucleophile → electrophile.
ExampleNucleophilic Substitution (Halogenoalkanes and OH⁻)
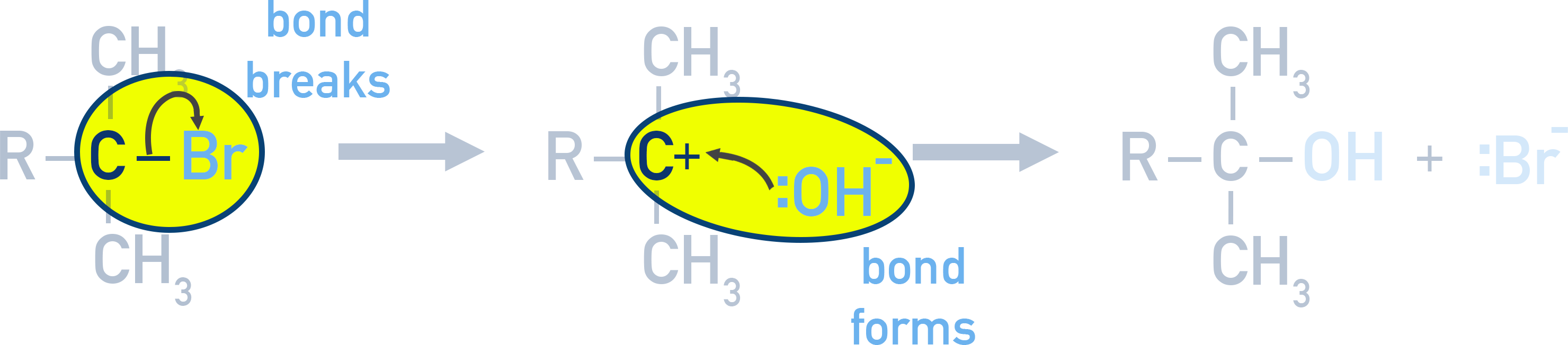
- Curly arrow shows the C-Br bond breaking, with Br⁻ leaving.
- Nucleophile (OH⁻) attacks positively charged carbon and curly arrow shows the lone pair forming a new bond.
- Nucleophile (OH⁻) attacks positively charged carbon and curly arrow shows the lone pair forming a new bond.
Electron Displacement Effects in Covalent Bonds
Permanent effects:
- Inductive effect: σ-electron shift due to electronegativity.
- Resonance effect: π-electron delocalisation across conjugated systems.
Temporary effect:
- Electromeric effect: Complete shift of π-electrons in the presence of an attacking reagent (only temporary).
Inductive Effect
When a covalent bond forms between atoms with different electronegativities, the shared electron pair is pulled more toward the more electronegative atom, creating a polar covalent bond.
Example: Chloroethane (CH₃CH₂Cl)
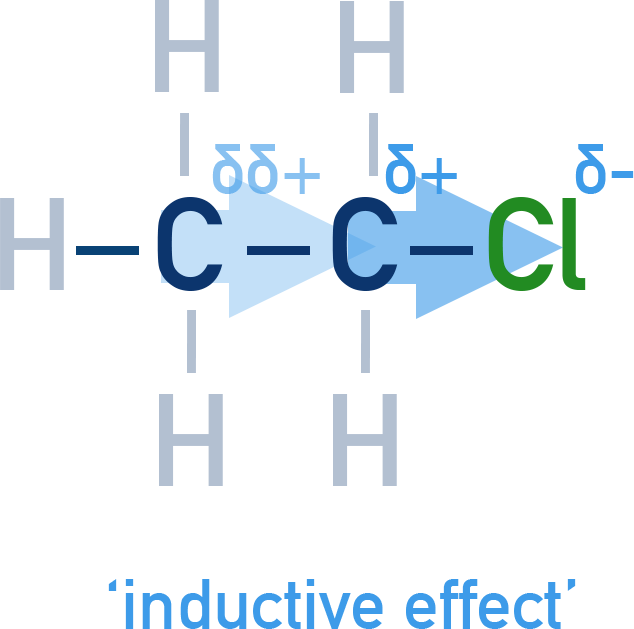
The C–Cl bond is polar due to chlorine's high electronegativity.
Electrons are pulled toward Cl, creating:
- δ⁺ on carbon-1 (next to Cl)
- δ⁻ on Cl
The positive charge (δ⁺) on carbon-1 pulls electron density from carbon-2, which also becomes slightly δ⁺ (δδ⁺), though weaker.
Effect Propagation:
This pull of electron density through sigma (σ) bonds is called the inductive effect. It is transmitted along the carbon chain, but its strength drops sharply after 2–3 bonds.
Types of Substituents:
Based on how they influence electron density, substituents are classified as:
- Electron-Withdrawing Groups (−I Effect):
- Pull electrons towards themselves
- Examples: Halogens (Cl, Br), Nitro (–NO2), Cyano (–CN), Carboxy (–COOH), Ester (COOR), Aryloxy (–OC6H5)
- Electron-Donating Groups (+I Effect):
- Push electrons toward the chain
- Examples: Methyl (–CH3), Ethyl (–CH2CH3)
Resonance Structure
Some molecules (e.g. benzene, nitromethane) cannot be represented by a single Lewis structure. Instead, they are represented by multiple resonance structures.
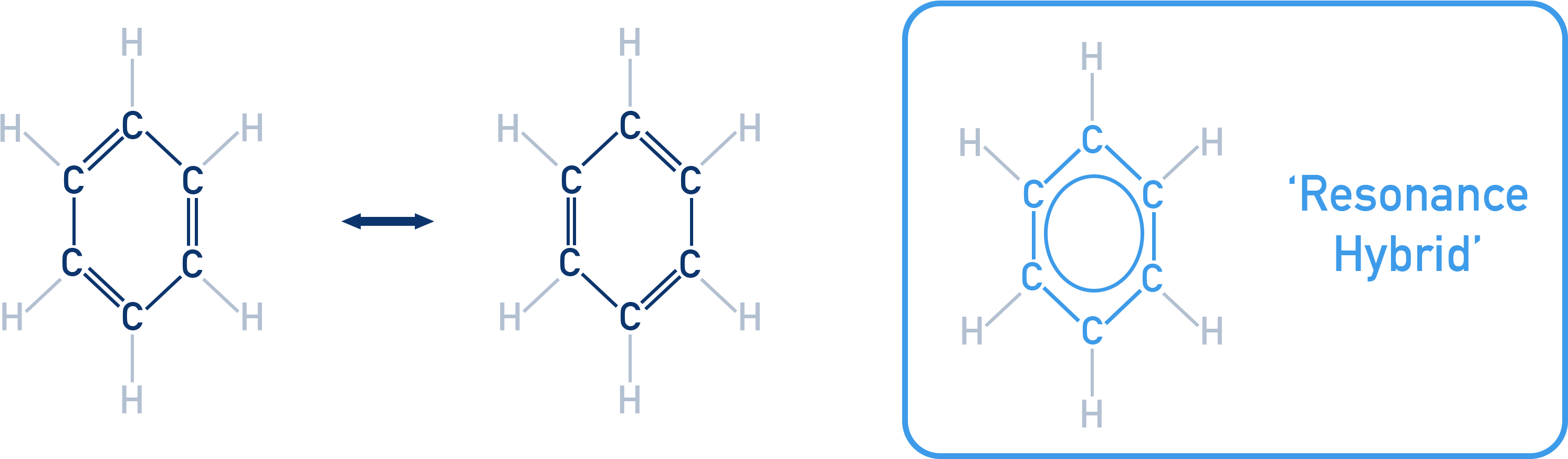
The actual molecule is a resonance hybrid with delocalised electrons.
Rules for resonance:
- Same atomic positions.
- Same number of unpaired electrons.
- Structure with more covalent bonds, full octets, and less charge separation is more stable.
Resonance energy = extra stability due to delocalisation.
Resonance Effect (or Mesomeric Effect)
Resonance involves the delocalisation of π-electrons or lone pairs through a conjugated system.
Types:
Positive resonance (+R) effect (electron donation):
For Example NH₂, OH, or halogens bonded to a benzene ring.
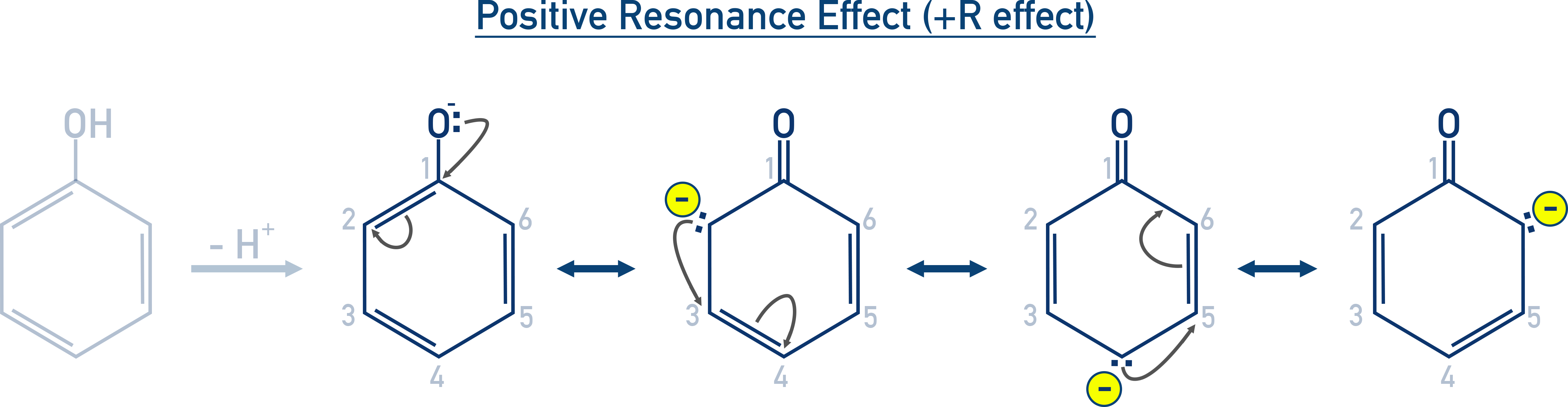
Negative resonance (–R) effect (electron withdrawal):
For Example NO₂, CN, COOH or CHO groups bonded to a benzene ring.

Affects reactivity and stability of molecules.
Electromeric Effect (E Effect)
A temporary effect seen in molecules with double or triple bonds. π-electrons shift completely to one atom upon attack by a reagent.
Types:
Positive electromeric (+E) effect:
Electrons move toward the attacking reagent (e.g. alkene + H⁺).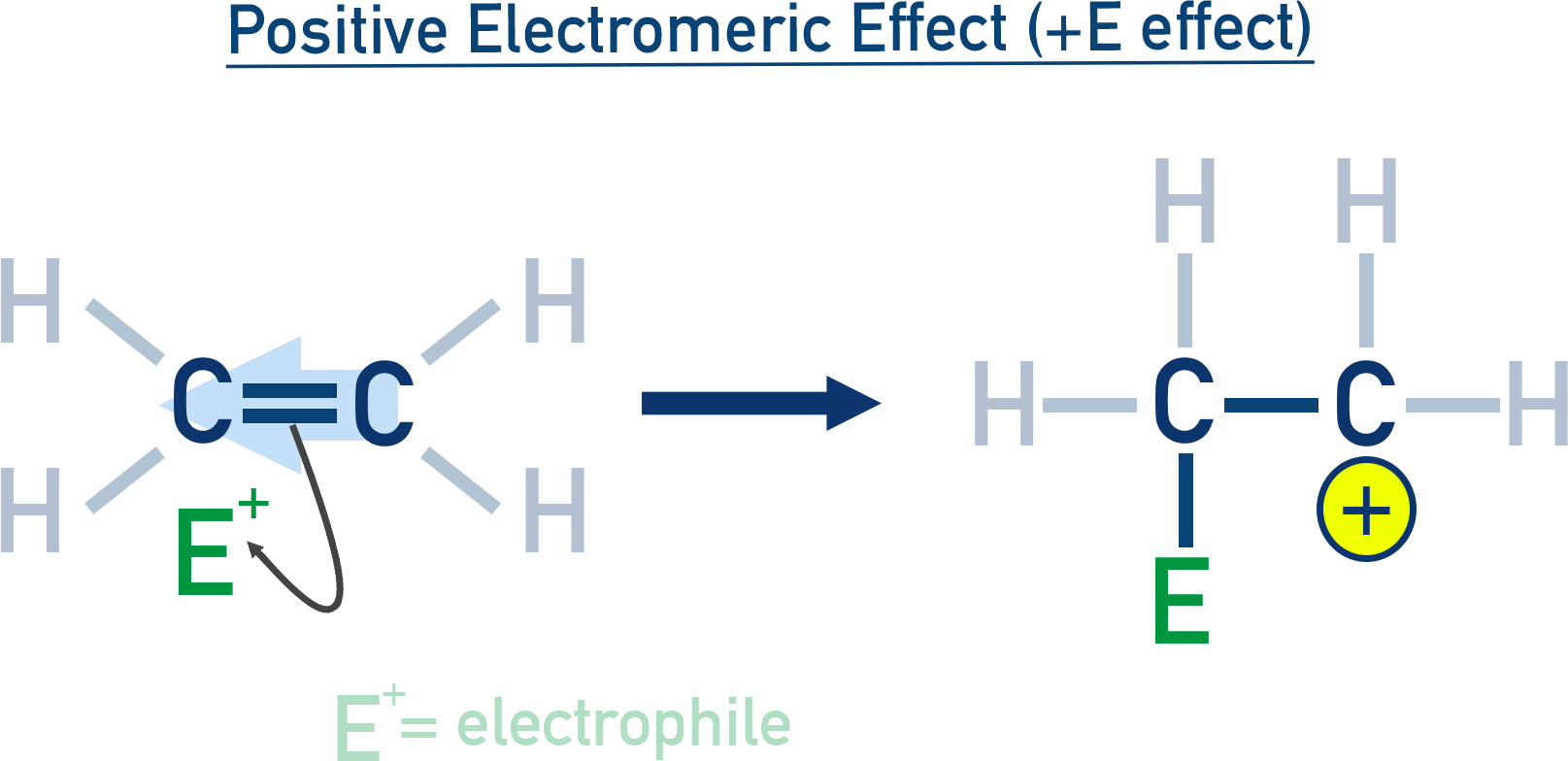
Negative electromeric (–E) effect:
Electrons move away from the attacking reagent (e.g. alkene + CN⁻).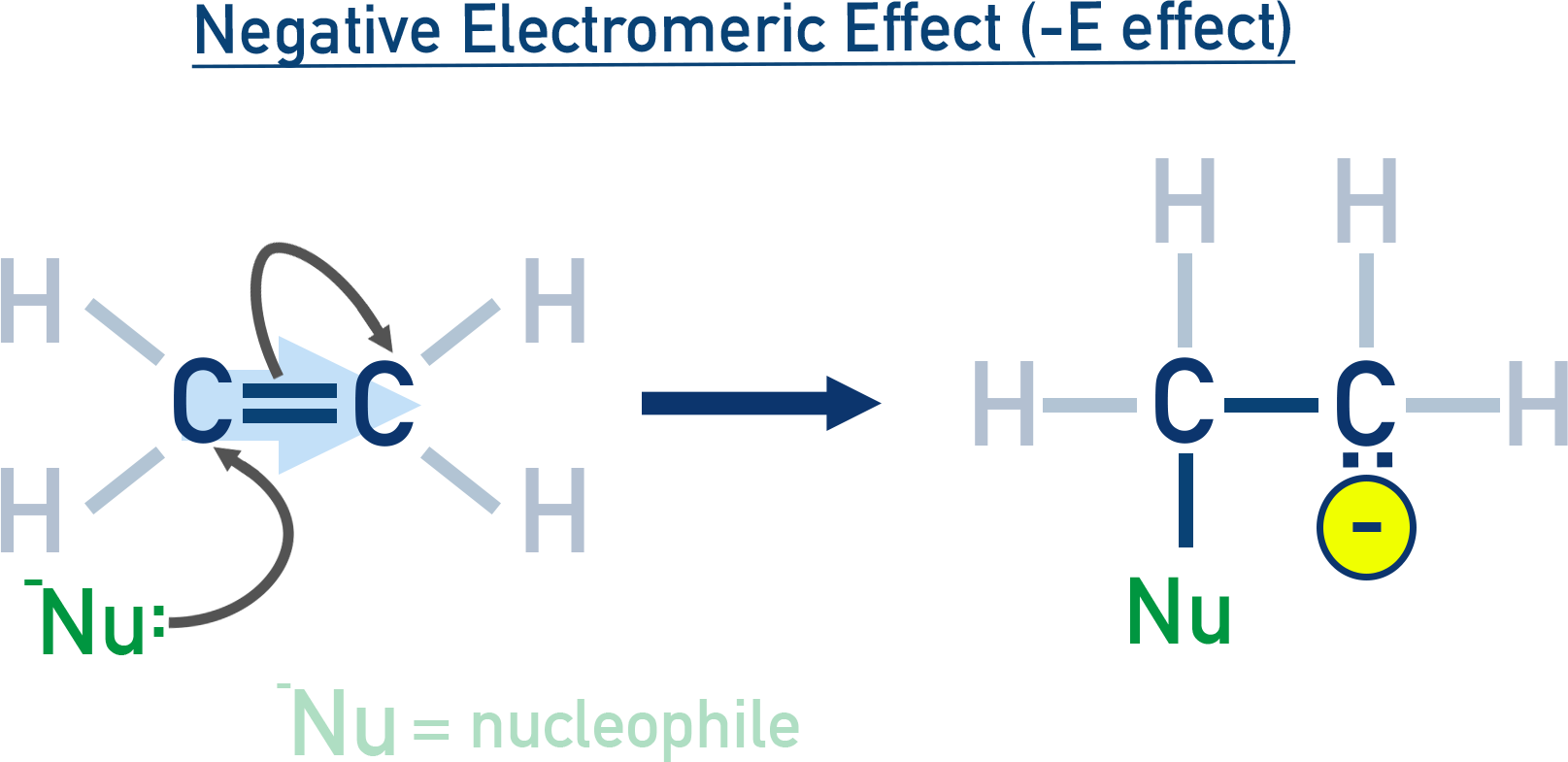
Operates only during the presence of an attacking species.
Hyperconjugation
Hyperconjugation is a general stabilising interaction.
Delocalisation of electrons:
In hyperconjugation, the electrons from the C–H bond of the alkyl group interact with an adjacent unsaturated system or a carbocation center. This delocalisation stabilises the molecule.
Example: Ethyl Carbocation (CH3–CH2+)
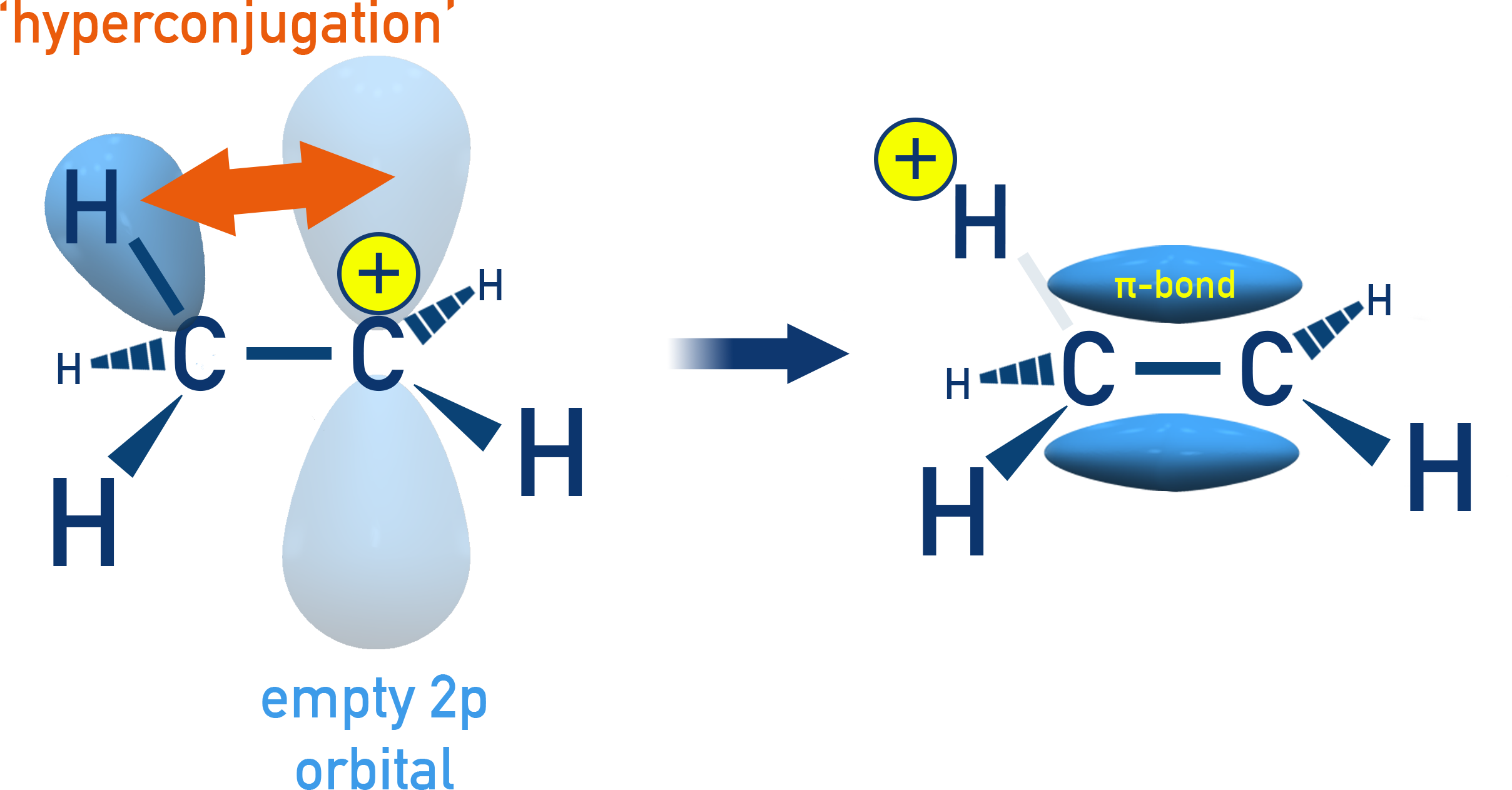
The C–H bond in the methyl group aligns with the empty p-orbital of the positively charged carbon. The electrons in this C–H bond can delocalise into the empty orbital, which helps in dispersing the positive charge.

Effect on Carbocation Stability:
More alkyl groups mean more C–H bonds available for hyperconjugation, thus more stabilisation.
The order of carbocation stability due to hyperconjugation is:
CH₃⁺ < CH₃–CH₂⁺ < (CH₃)₂CH⁺ < (CH₃)₃C⁺
(methyl < primary < secondary < tertiary)
Hyperconjugation in Alkenes:
Hyperconjugation also occurs in alkenes and alkyl-substituted aromatic compounds (alkylarenes).
For Example In propene (CH₃–CH=CH₂), the C–H bond electrons from the methyl group delocalise into the adjacent double bond, which increases stability.
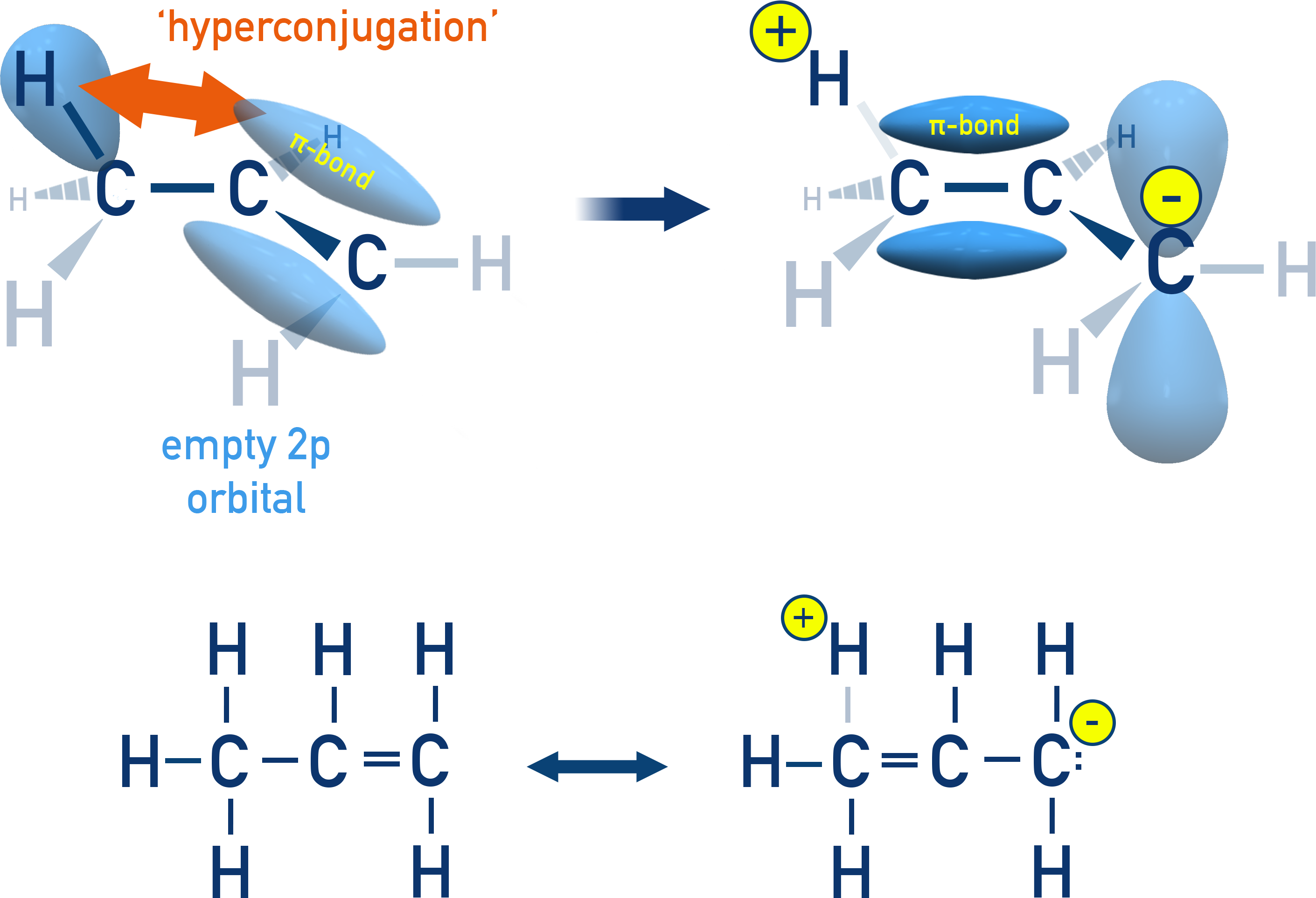
More alkyl groups = more hyperconjugation = more stability.
Helps explain stability order of carbocations:
CH₃⁺ < 1° < 2° < 3°
Types of Organic Reactions
- Substitution: One atom or group replaces another (e.g. CH₄ + Cl₂ → CH₃Cl).
- Addition: Atoms/groups are added across double or triple bonds (e.g. alkene + H₂).
- Elimination: Removal of atoms/groups to form multiple bonds (e.g. alcohol → alkene).
- Rearrangement: Reorganisation of atoms within the molecule to form isomers.
Summary
- Bond fission can be homolytic or heterolytic and generates key intermediates.
- Carbocation and radical stability increase with alkyl substitution due to +I and hyperconjugation.
- Inductive, resonance and electromeric effects govern electron movement and reactivity.
- Hyperconjugation stabilises carbocations and alkenes by σ to π or p delocalisation.
- Organic reactions proceed via substitution, addition, elimination or rearrangement pathways.
