Quick Notes - AS Organic Chemistry
Organic Introduction
Organic Chemistry - Introduction
- Empirical formula shows the simplest whole number ratio of elements within a molecule.
- Molecular formula shows the actual number of atoms of each element in a molecule.
- Structural formula shows how the atoms are arranged in a molecule.
- Displayed formula shows a drawing of the arrangement of atoms in a molecule.
- Skeletal formula shows the carbon backbone and functional groups within molecules, no carbon-hydrogen bonds are shown.
Carbon Chains
- Prefixes are used when naming organic molecules to show how many carbon atoms are bonded together successively (in a ‘chain’).
- meth = 1 carbon, eth = 2 carbons, pro = 3 carbons, but = 4 carbons, pent = 5 carbons, hex = 6 carbons, hept = 7 carbons, oct = 8 carbons, non = 9 carbons, dec = 10 carbons
- Hydrocarbons are molecules made up of only carbon and hydrogen atoms.
- Alkanes have single bonds between each carbon atom, all other available bonds are made to hydrogen atoms – they are called ‘saturated’ hydrocarbons.
- Alkenes have a double bond between two of their carbon atoms – they are called ‘unsaturated’ hydrocarbons.
- Alkly groups are carbon chain groups bonded to another carbon chain.
Functional Groups
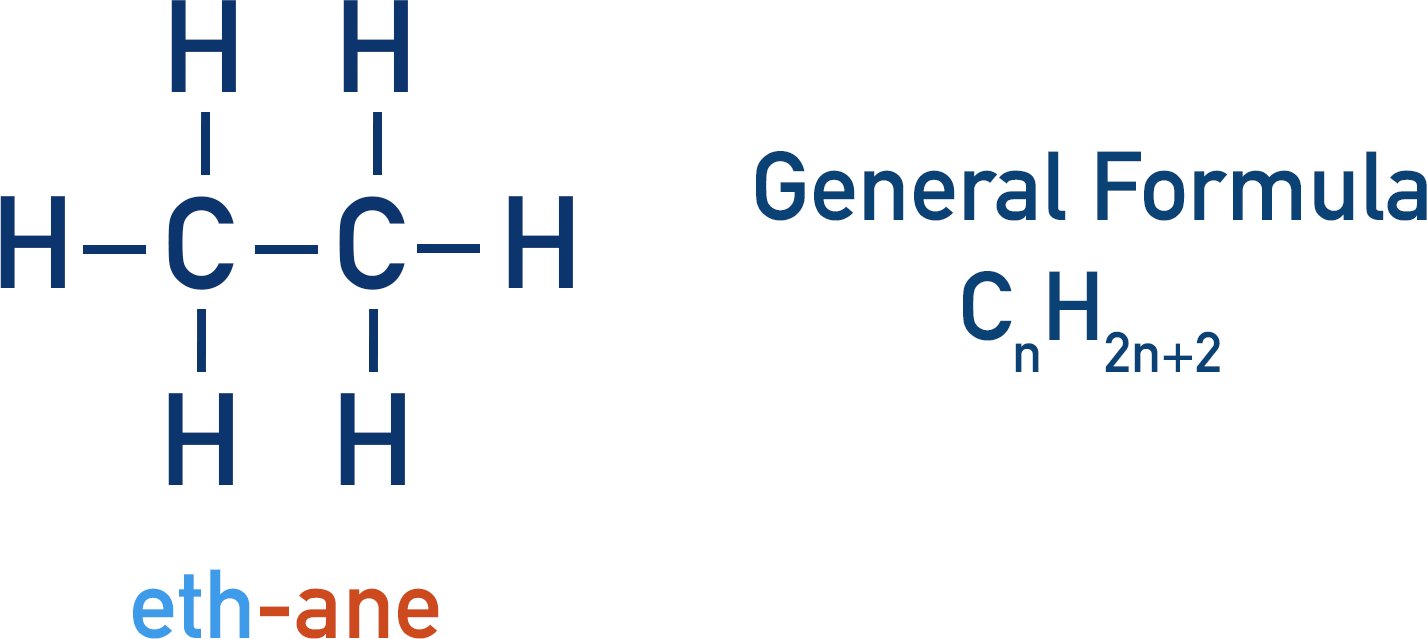
Alkane
Alkene

Halogenoalkane

Alcohol

Aldehyde

Ketone

Carboxylic Acid

Structural Isomerism
- Molecules that have the same molecular formula but different structures are called structural isomers.
- Chain isomers have different carbon chain arrangements to one another.
- Positional isomers have a functional group in different positions on their carbon chains.
Nomenclature
- Nomenclature is the process of naming compounds in organic chemistry.
- To name a compound, four basic rules are followed:
- Step 1 Identify the of longest carbon chain and choose the prefix (meth, eth...).
- Step 2 Identify the functional groups and choose the suffix (-ol, al…).
- Step 3 Identify the position of functional groups on the carbon chain (-1-ol, -3-ene.).
- Step 4 Place functional groups and alkyl chains in alphabetical order – if more than one.
Alkanes
Alkanes
- Alkanes are hydrocarbons in which the carbon atoms are bonded together with single covalent bonds.
- Alkanes are non-polar and do not dissolve in water or polar solvents.
- The combustion of alkanes releases large amounts of energy, making alkanes useful as fuels.
- Complete combustion of alkanes releases carbon dioxide, incomplete combustion releases carbon monoxide.
- Short chain hydrocarbons have low melting and boiling points (due to fewer intermolecular forces holding molecules together).
- Long chain hydrocarbons have high melting and boiling points (due to greater intermolecular forces holding molecules together).
Alkanes - Free Radical Substitution
- Covalent bonds can break in two ways:
- Heterolytic fission - bond breaks unevenly and both electrons from the bond go to one atom.
- Homolytic fission - bond breaks evenly and each bonded atom gets one electron, forming free-radicals.
- Free radicals are species that have an unpaired electron and are highly reactive.
- Halogens can react with alkanes in free-radical substitution reactions, the mechanism occurs as a ‘chain’ reaction with three stages:
- Initiation - U.V. light is needed to start the reaction and cause homolytic fission of the halogen molecule, creating two halogen radicals.
- Propagation – radical species react with the alkane and get substituted into the molecule, creating further radicals.
- Termination – two radical species combine to create a covalent bond and terminate the chain as the product is not a free radical.
Alkenes
Alkenes
- Alkenes are hydrocarbons in which two or more of the carbon atoms are bonded together with a double bond.
- A carbon double bond is made by the merging of a 2p-orbital from each carbon atom, creating an area of high electron density between the two atoms, called a ‘pi-bond’.
- Electron deficient species (electrophiles) are attracted to the electrons in the double bond, which makes alkenes more reactive than simple alkanes.
Alkenes - Stereoisomerism
- Carbon double bonds are unable to rotate freely like single bonds do – they have restricted rotation.
- Groups or atoms bonded to carbon atoms in a double bond are ‘locked’ into position, and there are two possible ways they can be arranged.
- Stereoisomerism occurs when two molecules have the same molecular and structural formula, but atoms within the molecules are arranged in space differently.
- Z and E notation is used to name alkene based stereoisomers.
- In Z isomers, the highest priority groups bonded to each carbon in the double bond are pointing in the same direction.
- In E isomers, the highest priority groups bonded to each carbon in the double bond are pointing in opposite directions.
- Cis and trans isomers are forms of Z and E isomers (respectively), but both carbons in the double bond are bonded to the same type of groups.
Alkenes - Electrophilic Addition Reactions
- Alkenes react by electrophilic addition reactions.
- Electron deficient species (electrophiles) are attracted to the pi-bonded electrons in a carbon double bond.
- In electrophilic addition, an electrophile causes the double carbon bond to break, and a new bond is formed between the electrophile and one of the carbon atoms.
- A carbocation (positively charged carbon atom) intermediate is formed that a negatively charged species forms a bond with.
- Primary carbocations are less stable than secondary and tertiary carbocations because they experience less of an inductive effect, meaning they aren’t as likely to form during electrophilic addition.
- Major and minor products of electrophilic addition reactions are determined by the stability of the intermediate carbocation that forms.
Alcohols
Alcohols
- Alcohols are hydrocarbons with a hydroxyl (OH) group bonded to a carbon in the chain.
- The O-H bond in alcohols is highly polar, meaning short chain alcohols (methanol and ethanol) are soluble in water.
- Longer chain alcohols are insoluble in water as the carbon chain (alkyl) is not polar.
- Hydrogen bonds can form between alcohol molecules, giving them higher melting and boiling points compared to alkanes with the same carbon chain.
- Alcohols can be primary (OH group bonded to a carbon bonded to only one other carbon), secondary (OH group bonded to a carbon bonded to two other carbon atoms) and tertiary (OH group bonded to a carbon bonded to three other carbon atoms).
Oxidation of Alcohols
- In organic chemistry, oxidation is a carbon atom gaining a bond to an oxygen atom and/or losing a bond to a hydrogen atom.
- To oxidise an alcohol, an oxidising agent (usually acidified potassium dichromate) is used and the alcohol is heated.
- Primary alcohols can be oxidised to an aldehyde, then to a carboxylic acid.
- To isolate the aldehyde, the products must be distilled from the reaction mixture.
- If a carboxylic acid is desired, the mixture must be heated under reflux conditions.
- Secondary alcohols can only be oxidised to form ketones.
- Fehling’s solution and Tollens’ reagent are used to distinguish between aldehydes and ketones.
- Aldehydes react to form a brick red precipitate with Fehling’s solution and a silver solid (silver mirror) with Tollens’ reagent. Ketones do not react with either.
- Teritary alcohols cannot be oxidised.
Halogenoalkanes
Halogenoalkanes - Nucleophilic Substitution Reactions
- Carbon-halogen bonds are highly polar.
- Due to the high electronegativity of halogens, the carbon becomes partially positively charged and the halogen partially negatively charged.
- Polarity of the carbon-halogen bonds decreases as you go down group 7.
- The carbon in a carbon-halogen bond is easily attacked by electron donating species (nucleophiles) that swap places with the halogen in nucleophilic substitution reactions.
- Key Reactions:
- Halogenoalkanes with sodium hydroxide in aqueous conditions form alcohols.
- Halogenoalkanes with ammonia in ethanolic conditions form amines.
- Halogenoalkanes with cyanide ions in ethanolic conditions form nitriles.
- Ethanolic conditions are needed instead of aqueous conditions, otherwise alcohols would form.
Halogenoalkanes - Elimination Reactions
- Halogenoalkanes can be converted to alkenes in an elimination reaction.
- By reacting halogenoalkanes with hydroxide ions in ethanolic conditions (anhydrous), an alkene and not an alcohol is formed.
- The reaction is carried out under reflux conditions and the hydroxide ion acts as a base (unlike in the hydrolysis of a halogenoalkane to form an alcohol).
