Video Tutorial Enthalpy Change
Quick Notes Enthalpy Change
- Enthalpy is a measure of the heat content of a substance or system. It has the units kJ mol-1.
- The actual enthalpy of a substance or system cannot be measured in chemistry, instead we measure how enthalpy changes during a reaction (enthalpy change, ΔH).
- During a chemical reaction energy can be exchanged between the reacting substance and its surroundings, this amount of energy is the enthalpy change of the reaction.
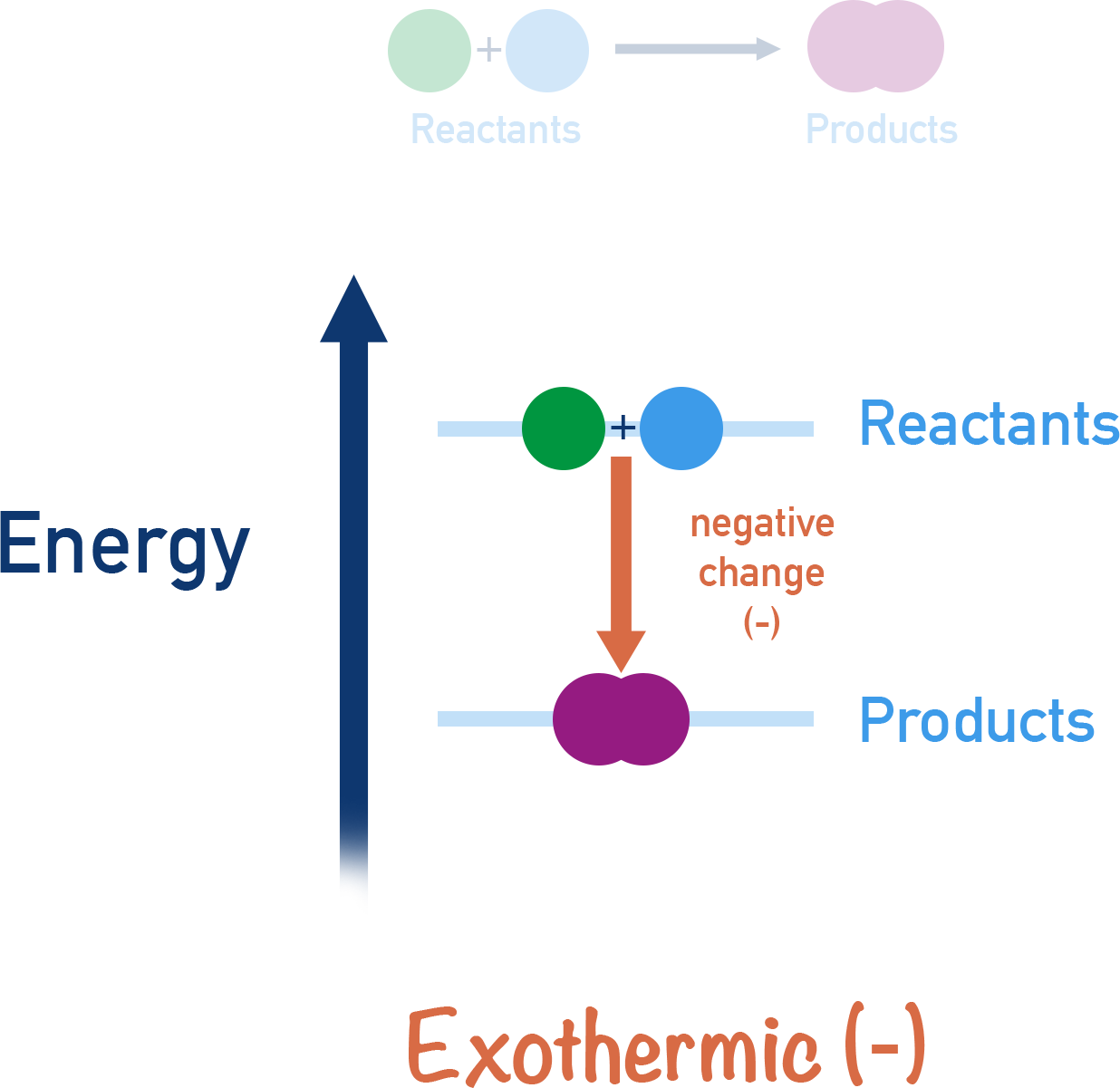
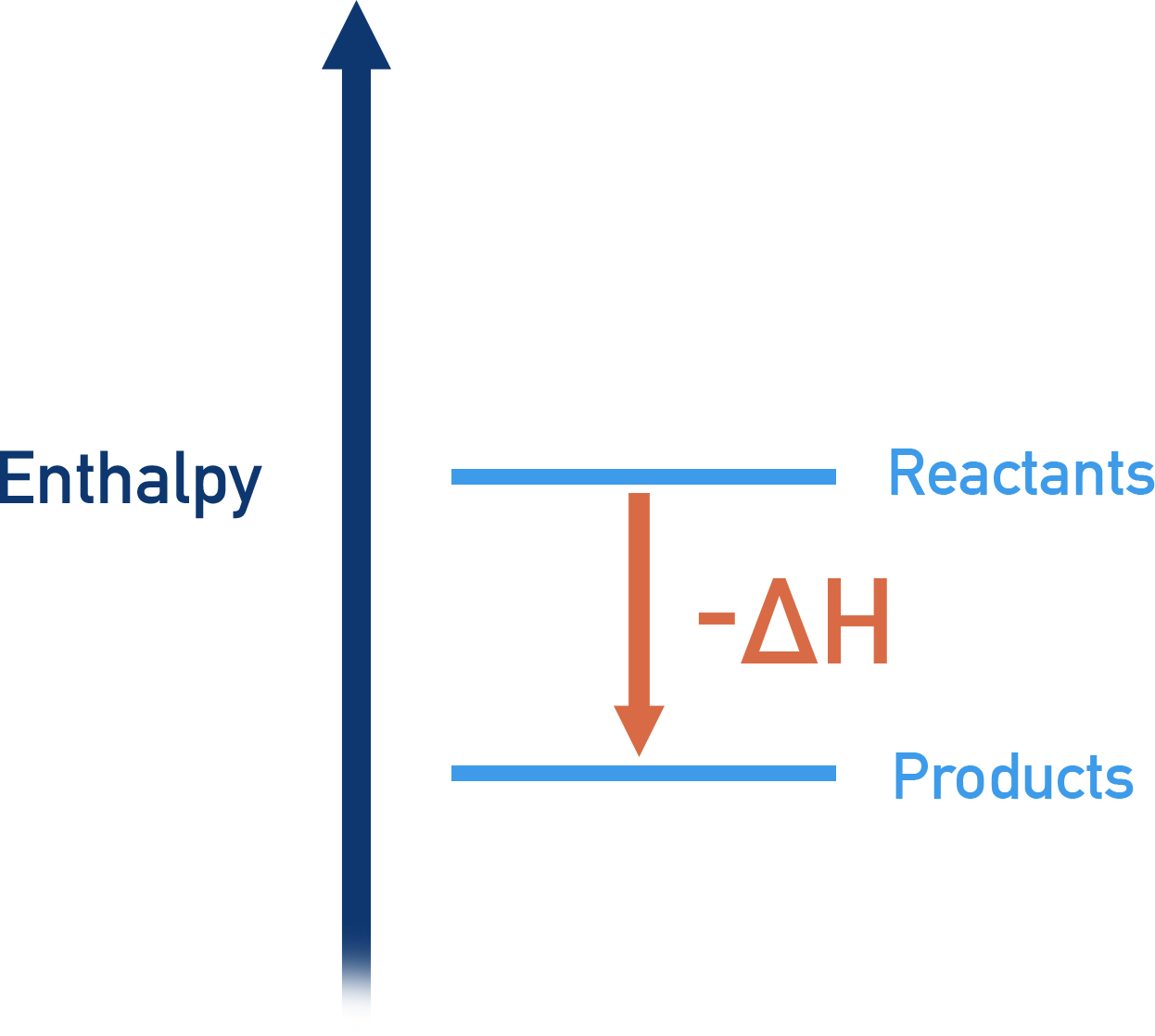
- Exothermic reactions – products are lower in energy than the reactants and the temperature of the surroundings increases.
- Exothermic reactions have negative enthalpy changes (-ΔH).
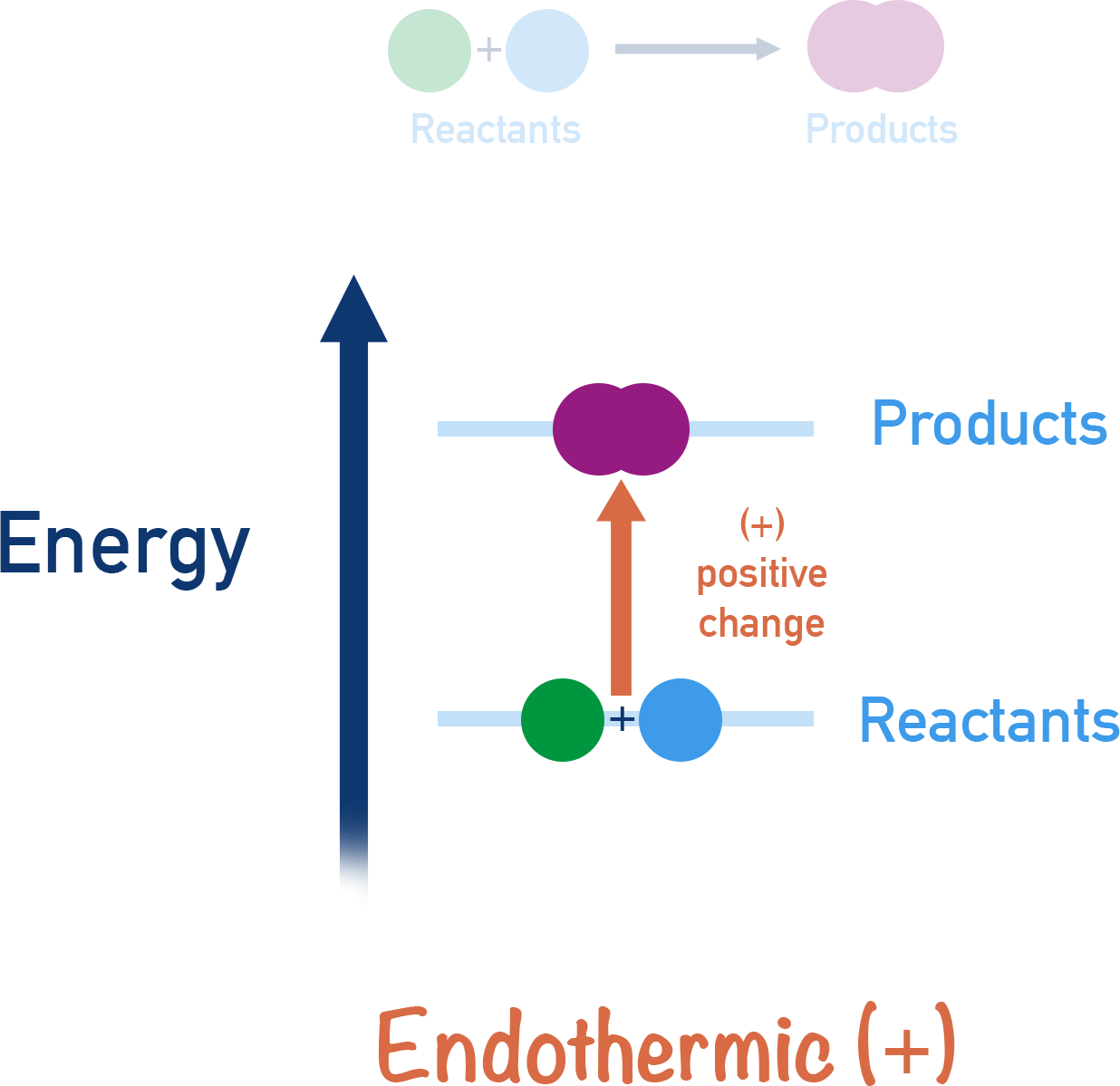
- Endothermic reactions – products are higher in energy than the reactants and the temperature of the surroundings decreases.
- Endothermic reactions have positive enthalpy changes (+ΔH).
- Standard enthalpy change refers to the thermal energy change that occurs during a reaction, where 1 mole of product is formed under standard conditions (101 kPa and 298K).
Full Notes Enthalpy Change
When chemical reactions happen, the products produced (nearly always!) have different energies to the reactants. This means energy has either been gained or lost by the reactants.
Energy cannot be created or destroyed, only transferred from one ‘store’ to another. During chemical reactions, energy is transferred between the reactants and the surroundings. The surroundings just refer to anything and everything around the reaction that is not actually reacting (for example, a gaseous atmosphere around the reaction).
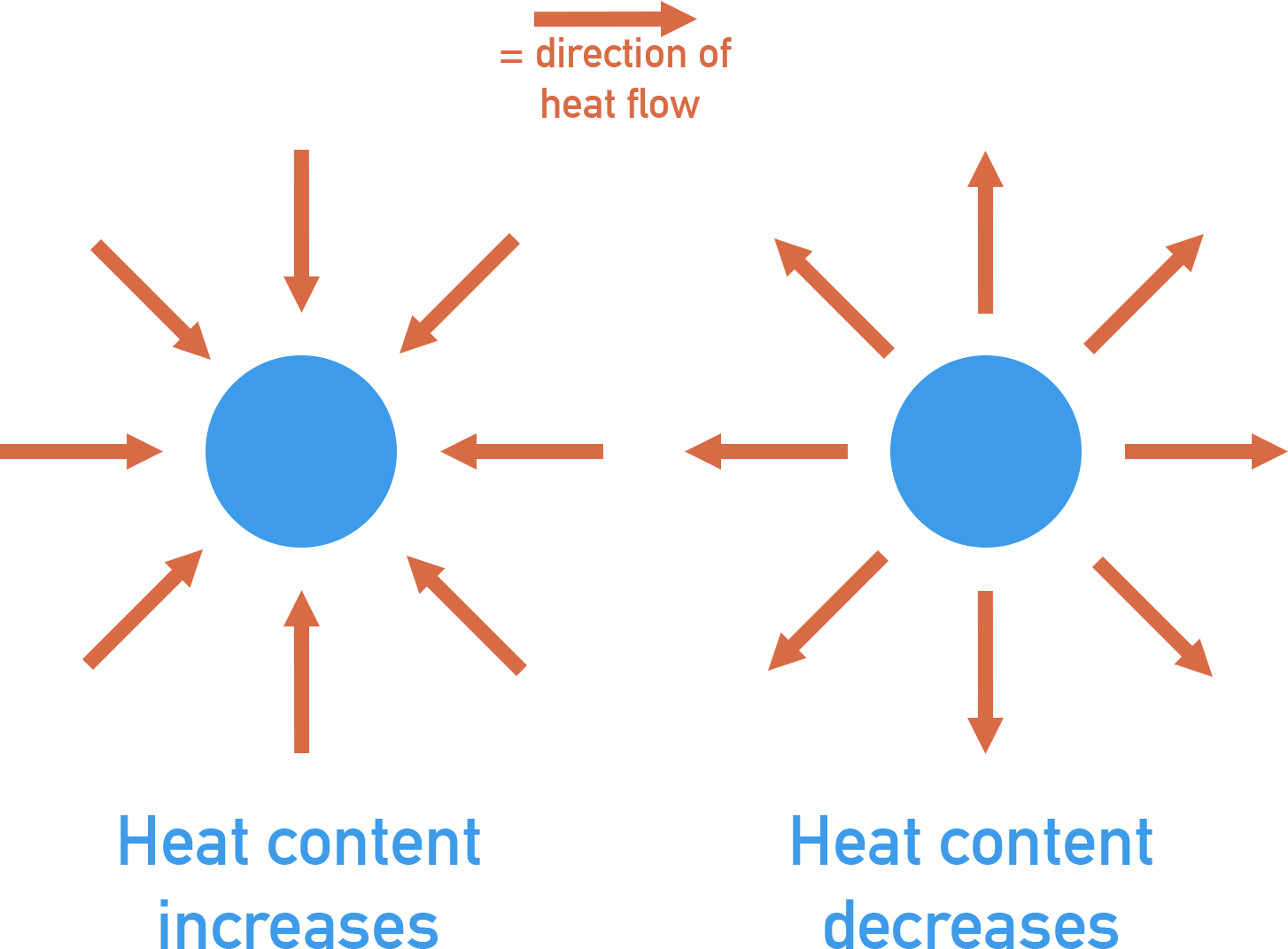
If the surroundings gain energy, the temperature of the surroundings increases. If the surroundings lose energy, the temperature of the surroundings decreases.
If energy is released in a reaction process, then the reaction is described as being exothermic.
If energy is absorbed in a reaction process, then the reaction is described as being endothermic.
Energy is transferred in the form of heat energy. In an exothermic reaction, heat energy is released from the reactants to the surroundings – the temperature of a reaction’s surroundings increases. In an endothermic reaction, heat energy is taken from the surroundings and absorbed by the reactants – the temperature of a reaction’s surroundings decreases.
The first law of thermodynamics tells us that the total energy in an isolated system remains constant. An isolated system is one where no energy or substances can get in or out (imagine a perfectly sealed container surrounded by a perfect vacuum).
In chemistry, we do not measure the actual amount of energy in a particular substance. Instead, we measure the energy change that occurs when substances react and form a new species. This energy change is called the enthalpy change(ΔH). This enthalpy change enables us to compare the energy of the reactants to the product(s).
Enthalpy change (ΔH) is measured as a change in temperature (heat energy) at a constant pressure.
If we want to measure and compare enthalpy changes for different processes, we have to make sure they are being measured in the same conditions.
We call these conditions ‘standard’. Standard enthalpy changes are enthalpy changes that have been measured at a particular pressure and stated temperature. The symbol for standard enthalpy change is ΔH⦵.
Standard conditions are 101KPa and 298K (25oC). This is NOT the same as room temperature and pressure, check your exam board and make sure you know the values required.
What is STP?
This is where things get even more confusing, STP refers to Standard Temperature Pressure and the values used are 273.15K and 100 KPa. Very simply, STP is used for ideal gas calculations (see Moles – Gases), whereas standard conditions are used for ‘everyday’ enthalpy changes and experiments at A-level.
Standard state refers to the 'usual' physical state of a substance under standard conditions.
We also need to know how much of our substance is reacting! The standard amount of substance used is always 1 mole.
For example:
The standard enthalpy of combustion (ΔH⦵c) is the change in enthalpy that occurs when 1 mole of a substance undergoes complete combustion under standard conditions.
The standard enthalpy of formation (ΔH⦵f) is the change in enthalpy that occurs when 1 mole of a substance is formed from its constituent elements in their standard states.
What is Enthalpy?
All substances have 'energy' stored up inside them.
In chemistry, we are interested in the potential energy stored up in bonds and the interactions between atoms and particles within a substance, sometimes refered to as chemical potential energy.
This energy can only ever leave a substance in the form of heat, meaning all substances are effectively 'stores' of thermal energy.
We describe this amount of 'stored energy' as enthalpy.
We can never know exactly how much energy is stored up in a given substance or system but we can measure how much energy either flows in or out during a reaction. We call this enthalpy change.
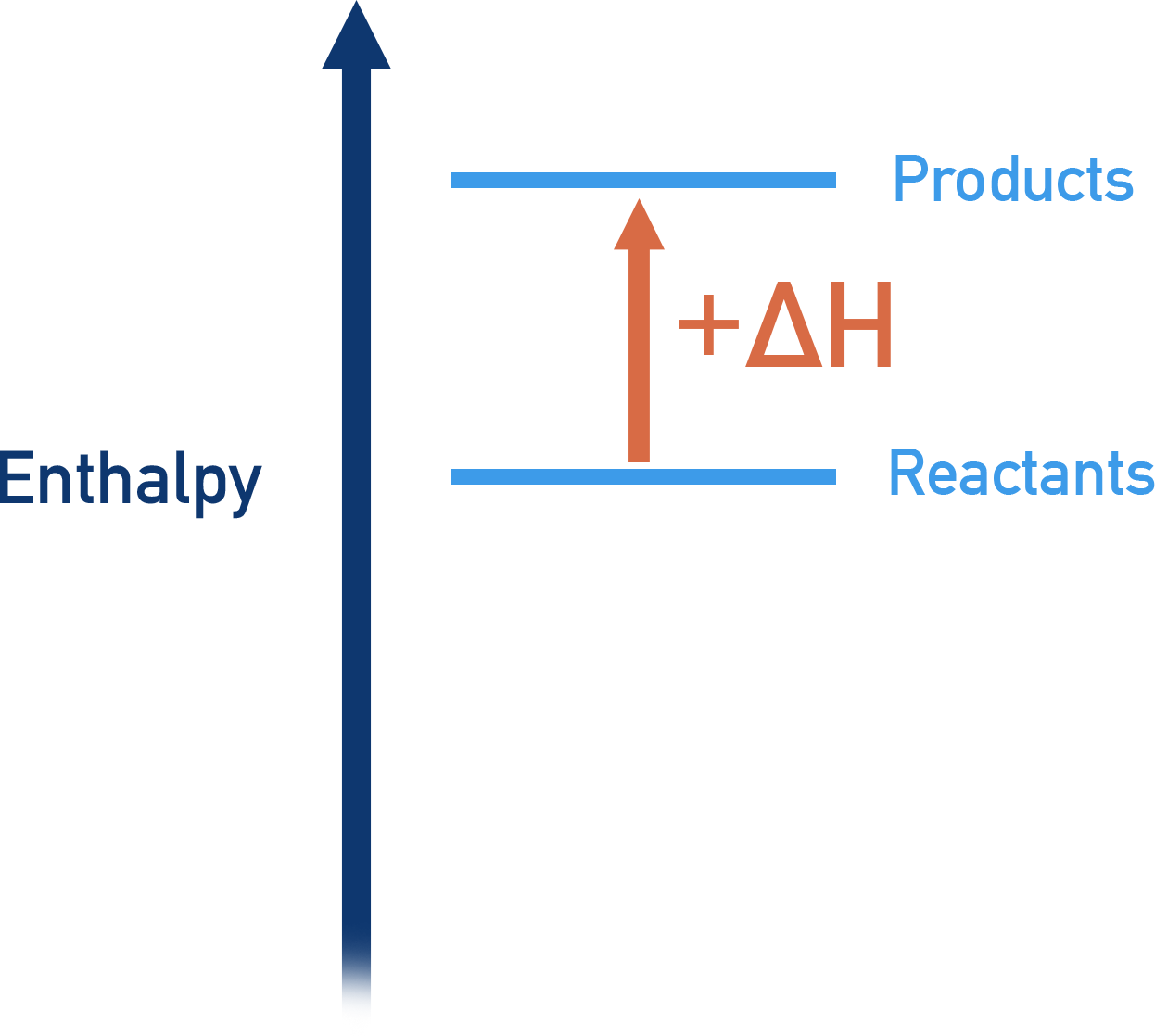
If energy flows into a substance during a reaction, the amount of stored up thermal energy (enthalpy) in the substance will increase - giving a positive enthalpy change (+ΔH).
If energy flows out of a substance during a reaction, the amount of stored up thermal energy (enthalpy) in the substance will decrease - giving a negative enthalpy change (-ΔH).
To measure enthalpy change, the temperature change of a reactions surroundings is used (see calorimetry). Enthalpy change must be measured under conditions of constant pressure as changes in pressure to a gaseous system can lead to a change in temperature, meaning temperature change of surroundings is based not only on the enthalpy change of reaction but also on the pressure change of the system.
We’ve launched our new site! 🎉
Course-specific notes with built-in search!
AP • A-Level (AQA • CIE • Edexcel • OCR) • IB • NCERT 11 + 12
over 750+ new pages and 3,500 images.
Visit the new homepage
