Quick Notes Addition Polymerisation
- Polymers are long chain molecules made of repeating units bonded together.
- Molecules that form repeating units are called monomers.
- Alkenes can undergo addition polymerisation to create polyalkenes.
- The double carbon bond can be opened to enable each carbon from the double bond to form new bonds with another repeating unit.
Full Notes Addition Polymerisation
Polymers are large molecules that are made up of repeating units. The repeating units themselves are made from simple molecules called monomers.
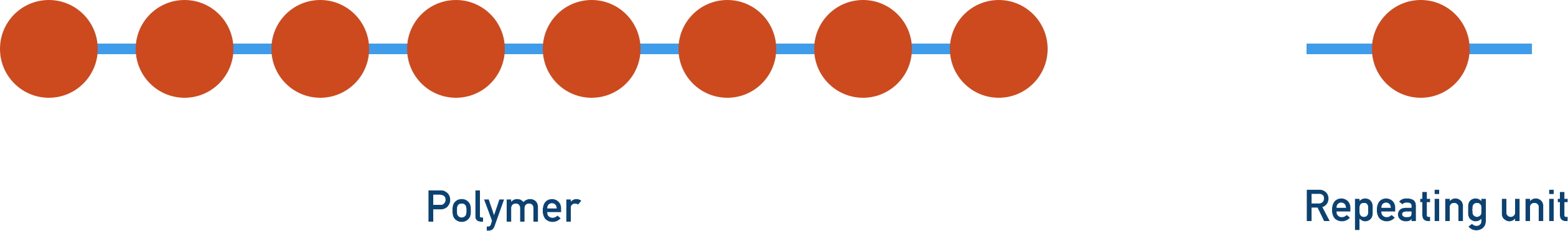
Addition Polymerisation
In addition polymerisation, monomers simply bond together with no extra reagents needed or products produced. For example, the addition polymerisation of ethene to produce polyethene:
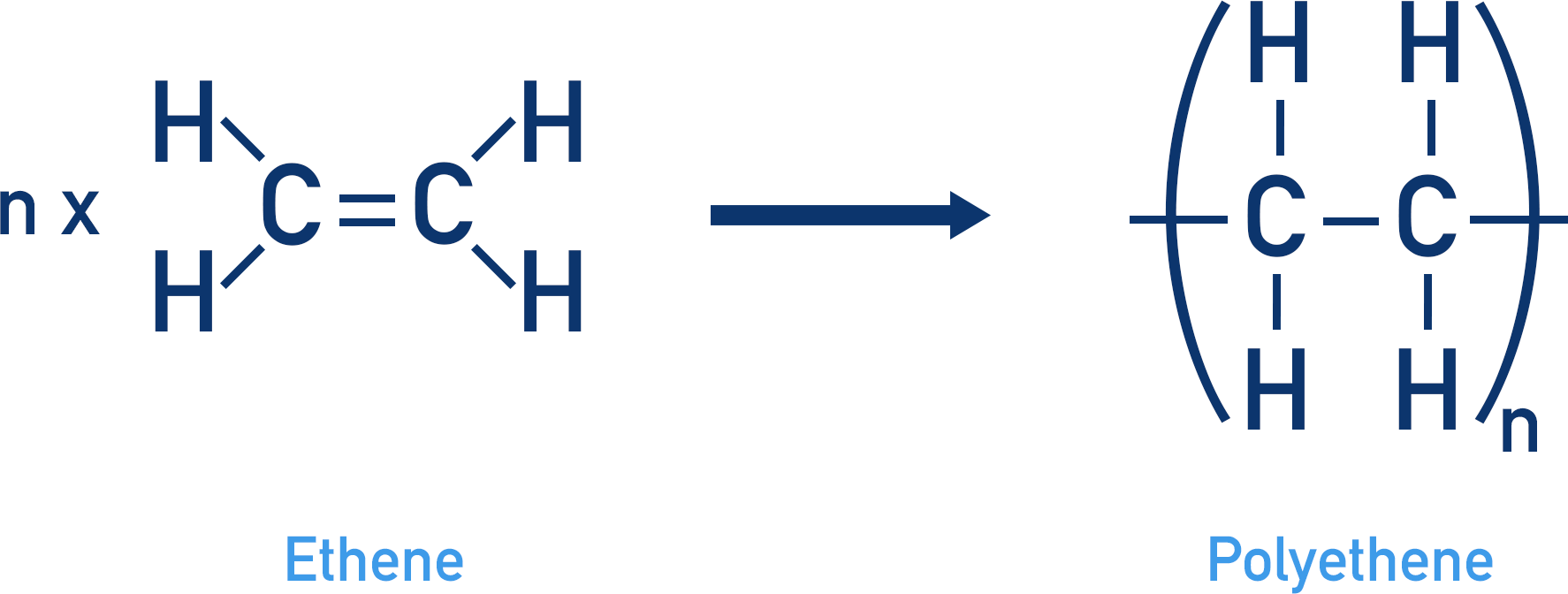
The ethene molecule contains one double carbon bond that can be ‘cleaved’ open, leaving a single bond between the carbon atoms and the ability for each carbon atom to form another bond. This new arrangement creates the repeating unit that will form the polymer.
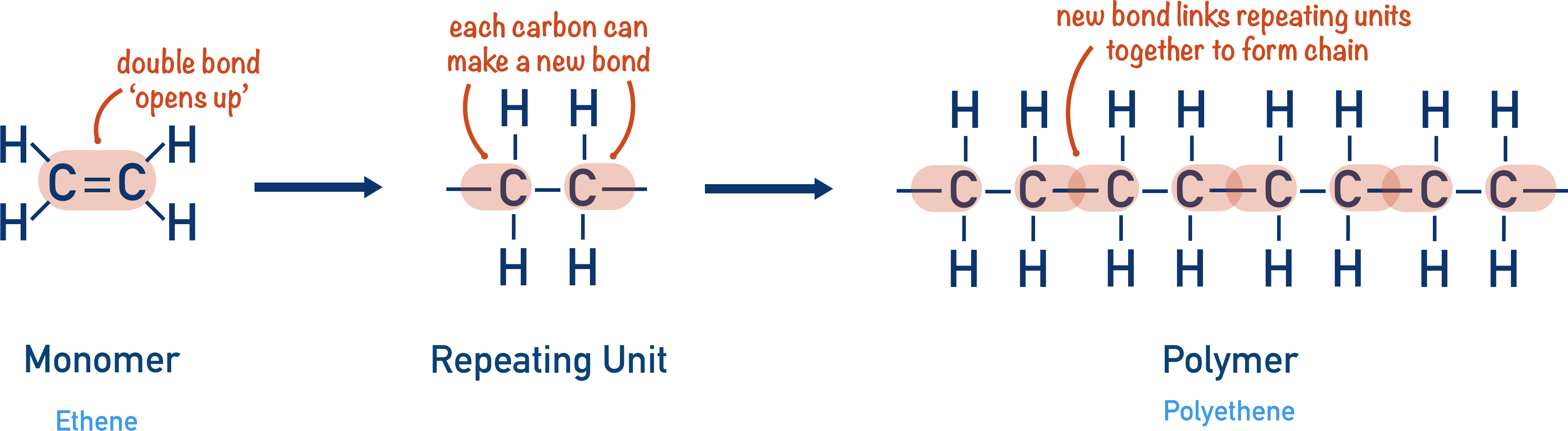
By bonding with another repeating unit, a simple chain is formed. No other reagents are needed and there are no other products produced. It is an efficient use of resources and the entire mass of ethene (or any alkene) that is used will be the same as the entire mass of the polymer (polyethene) formed.
