Video Tutorial Amino Acids (and Zwitterions)
Quick Notes Amino Acids and Proteins
- Amino acids are a collection of different organic molecules that all contain an amine group and a carboxylic acid group.
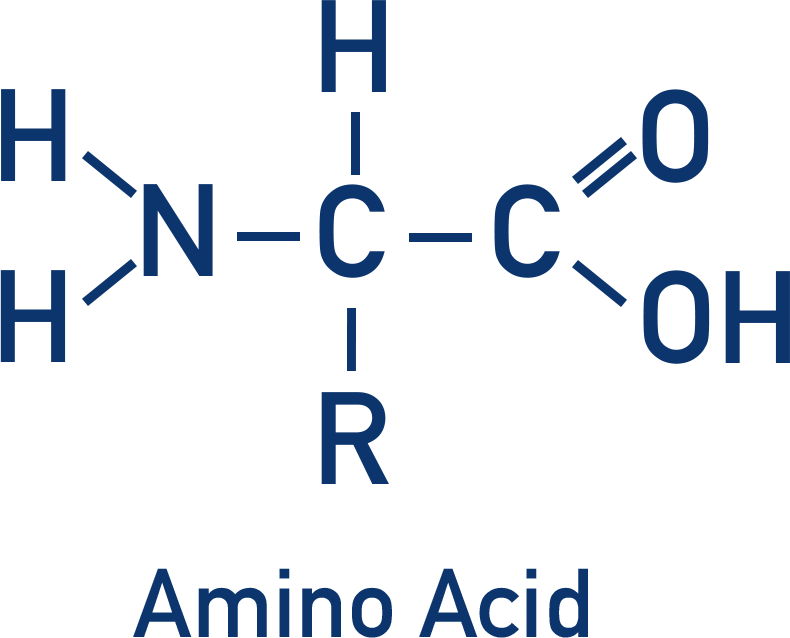
- Amino acids join together to form proteins.
- The amine group in an amino acid can accept a proton (basic) to become a positive ion.
- The carboxylic acid group in an amino acid can donate a proton (acidic) to become a negative ion.
- Different amino acids lose or gain protons at different pHs.
- A zwitterion is an amino acid that has lost a proton from its carboxylic acid group but gained a proton at its amine group – forming negatively and positively charged groups, but no overall charge.
- Zwitterions are formed at an amino acid’s isoelectric point.
Full Notes Amino Acids and Proteins
Amino acids are the building blocks of life. Living organisms are made of proteins that are themselves polymers of amino acids.

When amino acids are linked together, proteins are formed. There are only 22 naturally occurring amino acids (21 used by the human body) and yet each human has over 6,000,000 different types of protein. By changing the order of the amino acids linked together, the shape and structure of the polymer formed is also changed, producing a new protein.
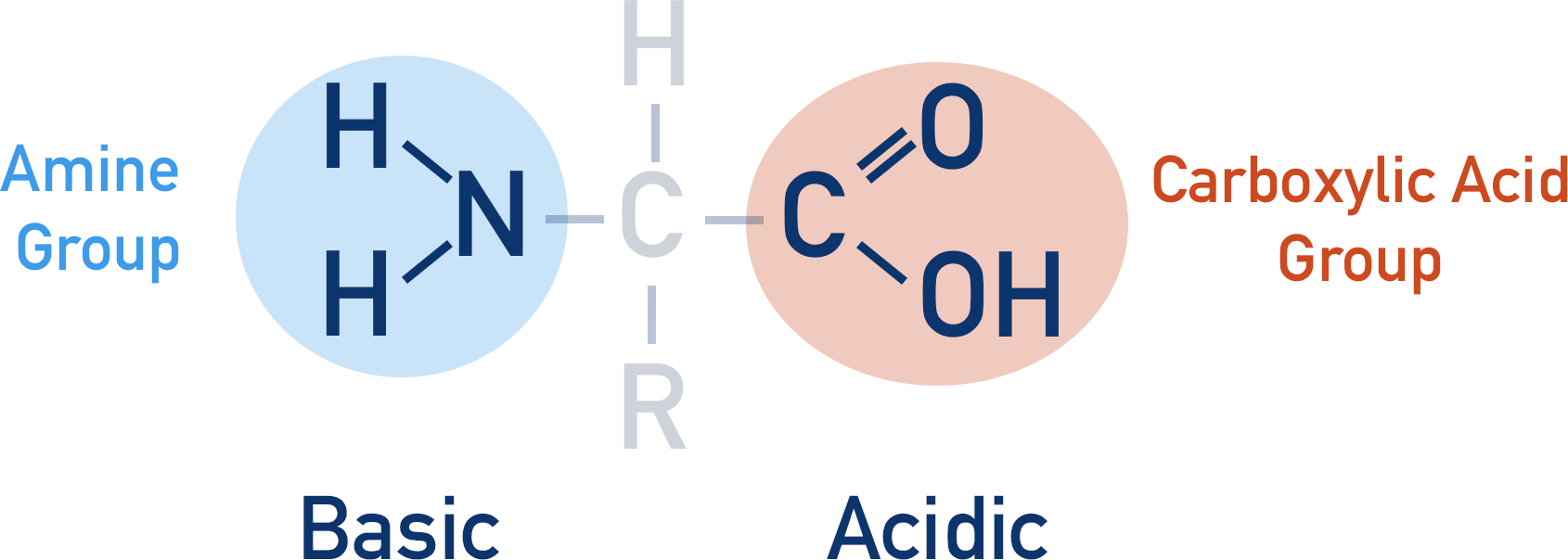
From a chemistry view, amino acids are interesting organic molecules as they have both an amine group and a carboxylic acid group. An amino acid can act as both an acid and a base.
Zwitterions
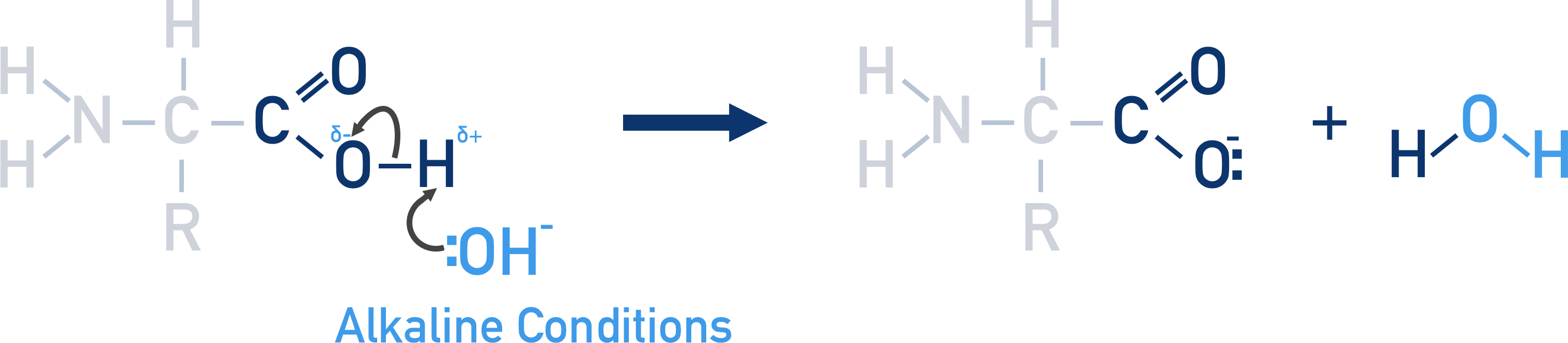
The carboxylic acid group in an amino acid can release a proton into solution and become a negative ion.
The amine group in an amino acid can act as a base – it can accept a proton in a solution and become a positive ion.
If an amino acid is placed into a strong alkaline solution, the carboxylic acid will lose a proton and the amino acid will become negatively charged.
If an amino acid is placed into a strong acidic solution, the amine group will accept a proton and the amino acid will become positively charged.
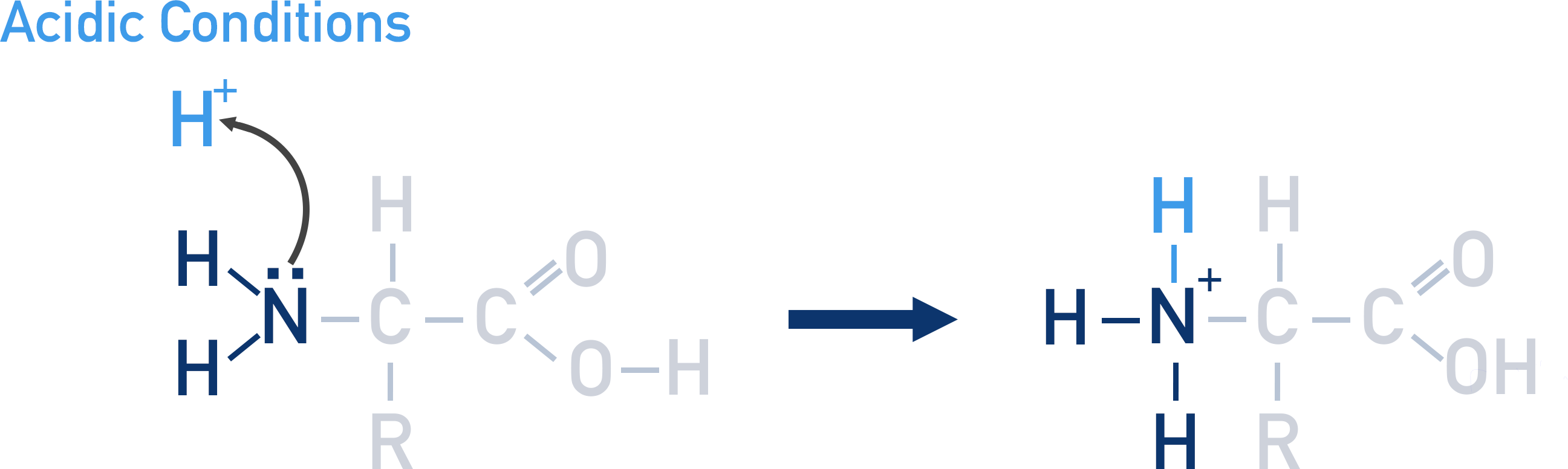
All amino acids have different strengths of acidity and basicity, meaning the carboxylic acids and amine groups donate and accept protons at different pHs.
At just the right pH, a proton released from the carboxylic acid can be accepted by the amine group.
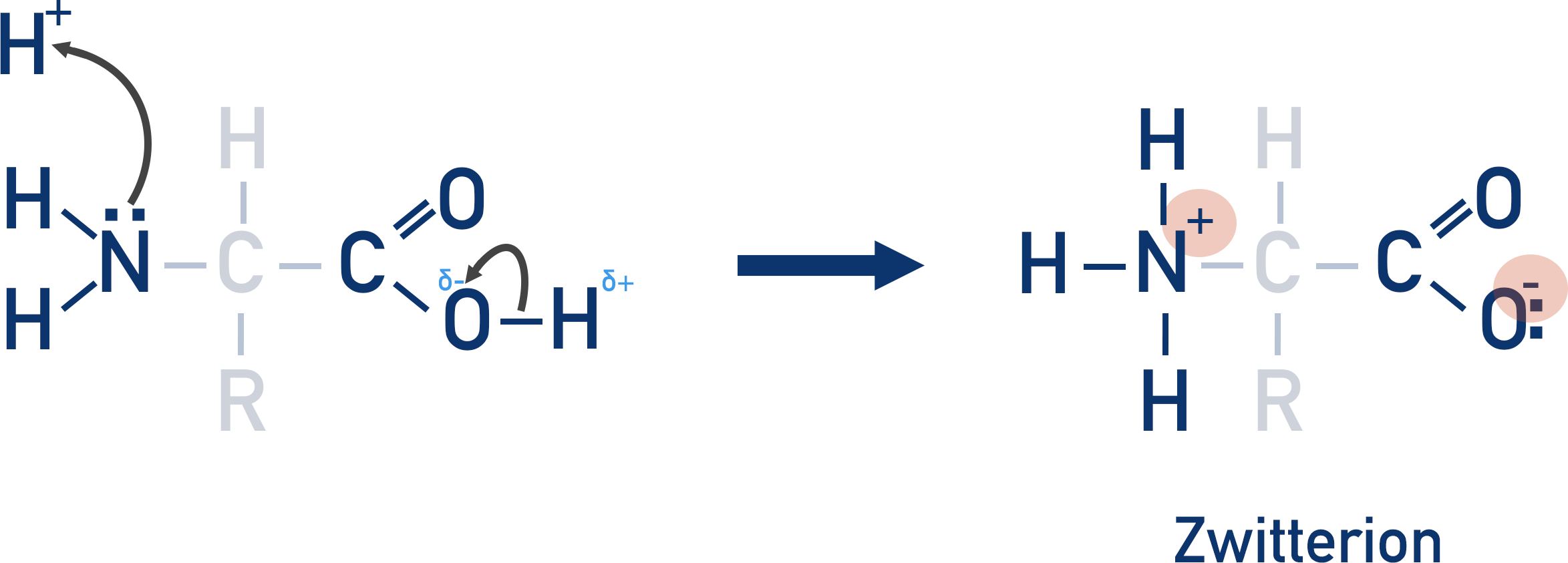
There is no overall charge to the amino acid. This is called a zwitterion and is only formed at a specific pH for each amino acid. The pH at which a zwitterion is formed is called the isoelectric point.
We’ve launched our new site! 🎉
Course-specific notes with built-in search!
AP • A-Level (AQA • CIE • Edexcel • OCR) • IB • NCERT 11 + 12
over 750+ new pages and 3,500 images.
Visit the new homepage
