Quick Notes - AS Physical Chemistry
Atomic Structure
Structure of an Atom
- Atoms are the smallest units of an element.
- Atoms are made of sub-atomic particles:
- protons (relative charge +1, relative mass 1)
- neutrons (relative charge 0, relative mass 1)
- electrons (relative charge -1, relative mass 1/1840)
- The number of protons an atom has is called its ‘atomic number’.
- The centre of an atom is called a nucleus; it contains just protons and neutrons, making the nucleus positively charged.
- Electrons are attracted to the positive charge of a nucleus and exist in orbitals around the nucleus.
- Atoms can lose or gain electrons to become charged ions.
Electron Orbitals
- Electrons are negatively charged, so they are attracted to the positive charge of a nucleus (electrostatic attraction).
- The closer an electron is to a nucleus, the lower its energy.
- All electrons in an atom want to get as close to the nucleus as possible, but they repel each other.
- This forces electrons to exist in set regions called orbitals.
- One orbital can hold two electrons (one pair).
- Different orbitals have different shapes and energies. Orbital types are labelled with letters – s, p, d and f.
- Shells describe how far away an electron is from a nucleus, and therefore its energy.
- Shell 1 is the closest shell to the nucleus, shell 2 the next closest, etc.
- Due to different shapes, orbitals within a shell can have different energies and form sub-shells.
- Shells and sub-shells are filled successively – lowest available energy sub-shells and orbitals are filled first.
Electron Configurations
- Electrons occupy orbitals of lowest energy around a nucleus.
- Higher energy orbitals are only occupied if all lower energy orbitals are already full.
- Electron configurations show how electrons are arranged around a nucleus within an atom or ion.
- The first number shows the shell the electrons are in.
- The letter shows the shape of the orbital the electrons are in.
- The second number shows the number of electrons in the orbital(s).
- Due to electron repulsion and orbital shapes, 4s orbitals are occupied before the 3d orbitals.
Moles (Amounts)
The Mole
- Atoms and particles are very small, so referring to actual number of atoms in a sample isn’t very practical.
- The mole is a unit used to refer to an amount of atoms or particles.
- Just like one dozen = 12 of something, one mole = 6.022 x 1023 of something.
- The number one mole refers to is known as Avogadro’s constant.
Relative Atomic Mass
- Relative atomic masses (Ar) are used to compare the masses of all elements on the periodic table.
- Relative atomic mass (Ar) is the weighted average mass of one atom of each element – compared to 1/12th the mass of one atom of carbon-12.
- one carbon-12 atom has six protons and six neutrons
- relative mass of one proton is one
- relative mass of one neutron is one
- relative mass of one atom of carbon-12 is twelve
- The mass of an atom is determined by the number of protons and neutrons it has.
- Protons and neutrons have (virtually) the same mass.
- The actual mass of protons and neutrons are too small to use when describing the mass of an atom, instead both particles are assigned a relative mass of one.
- Relative atomic mass is a weighted average that accounts for the abundance of each naturally occurring isotope of that element.
- Molar mass is the mass (g) of one mole’s worth of something (g mol-1).
Moles from Mass
- The number of moles in a sample can be calculated using the formula:
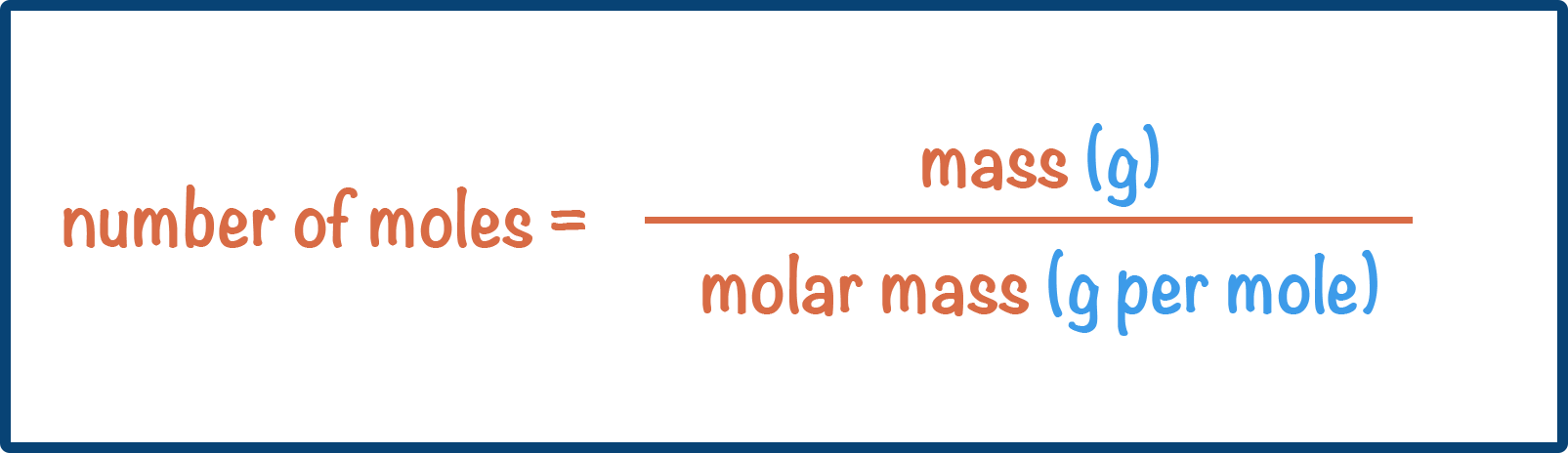
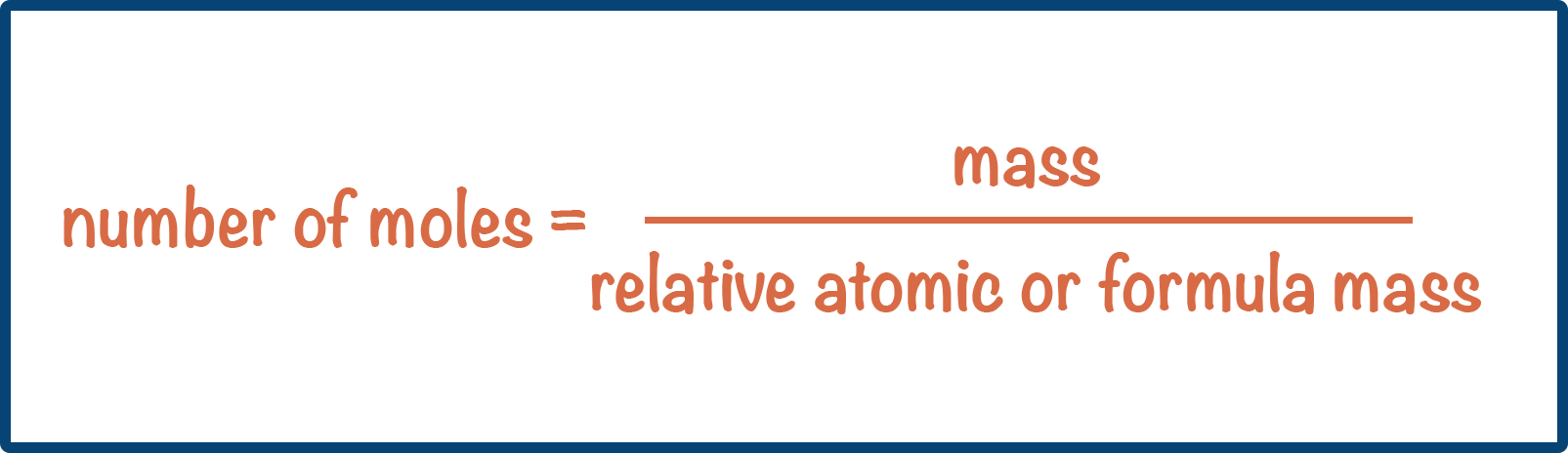
- The value of relative atomic mass of an element = value of molar mass (in grams per mol).
- The value of relative formula and molecular mass = value of molar mass (in grams per mol).
- This means moles can be calculated using:
Moles - Solutions
- The number of moles of a solute in a solution can be calculated using the formula:
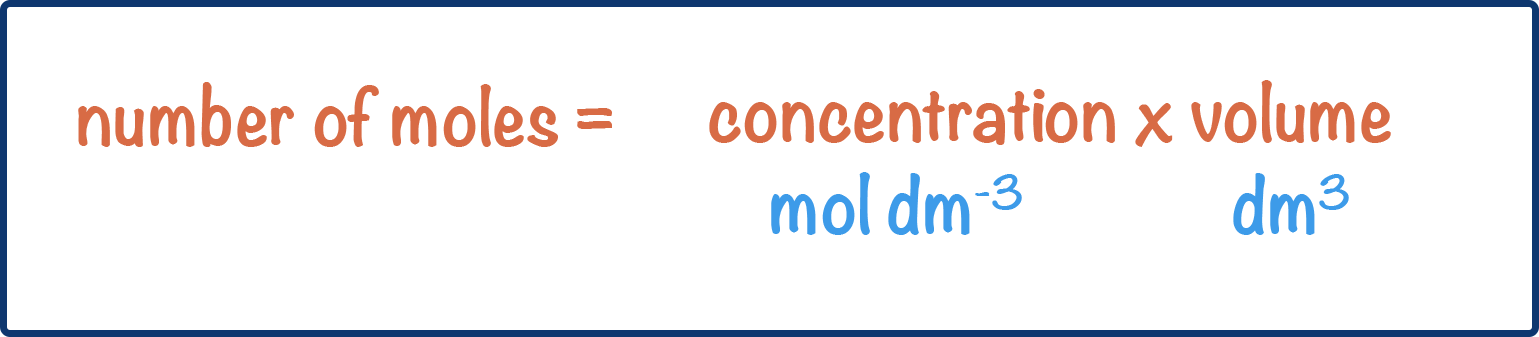
- Volume (of solution) is usually given in cm3 and must be converted into dm3.
- 1 dm3 is the same as 1000 cm3
- To convert dm3 to cm3 x 1000
- To convert cm3 to dm3 / 1000
- Concentration refers to how many moles of solute are in 1dm3 of solution (units are mol dm-3).
Moles - Gases
- The ideal gas equation is used to calculate the moles of gas present in a system if the volume, pressure and temperature are known.
- pV = nRT
- n = moles
- R = gas constant = 8.31 Jmol-1K-1
- V = volume (m3)
- p = pressure (Pa)
- T = temperature (K)
- pV = nRT
- The equation assumes that all gases behave in the same way and are not interacting with each other, they are described as ‘ideal gases’.
- At room temperature and pressure (293K and 101kPa), the ideal gas equation shows that 1 mole of any gas occupies a total volume of 24000cm3 (24dm3).
- At standard temperature and pressure (273K and 101kPa), the ideal gas equation shows that 1 mole of any gas occupies a total volume of 22400cm3 (22.4dm3).
Bonding
Bonding
- Bonds between atoms are called atomic bonds.
- Forces between molecules are called intermolecular forces.
- Intermolecular forces are broken and formed when molecular substances change state (solid, liquid and gas).
Covalent Bonding
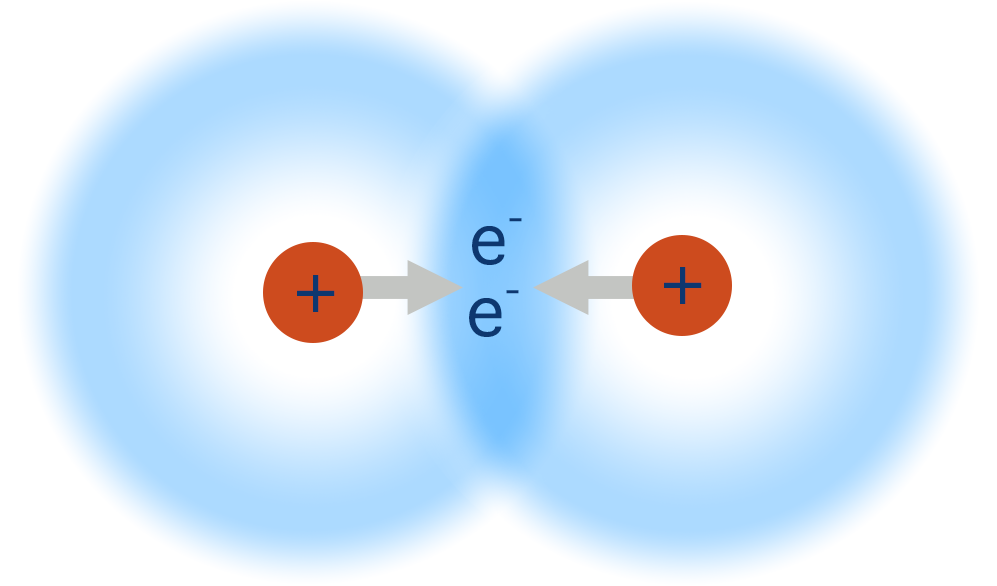
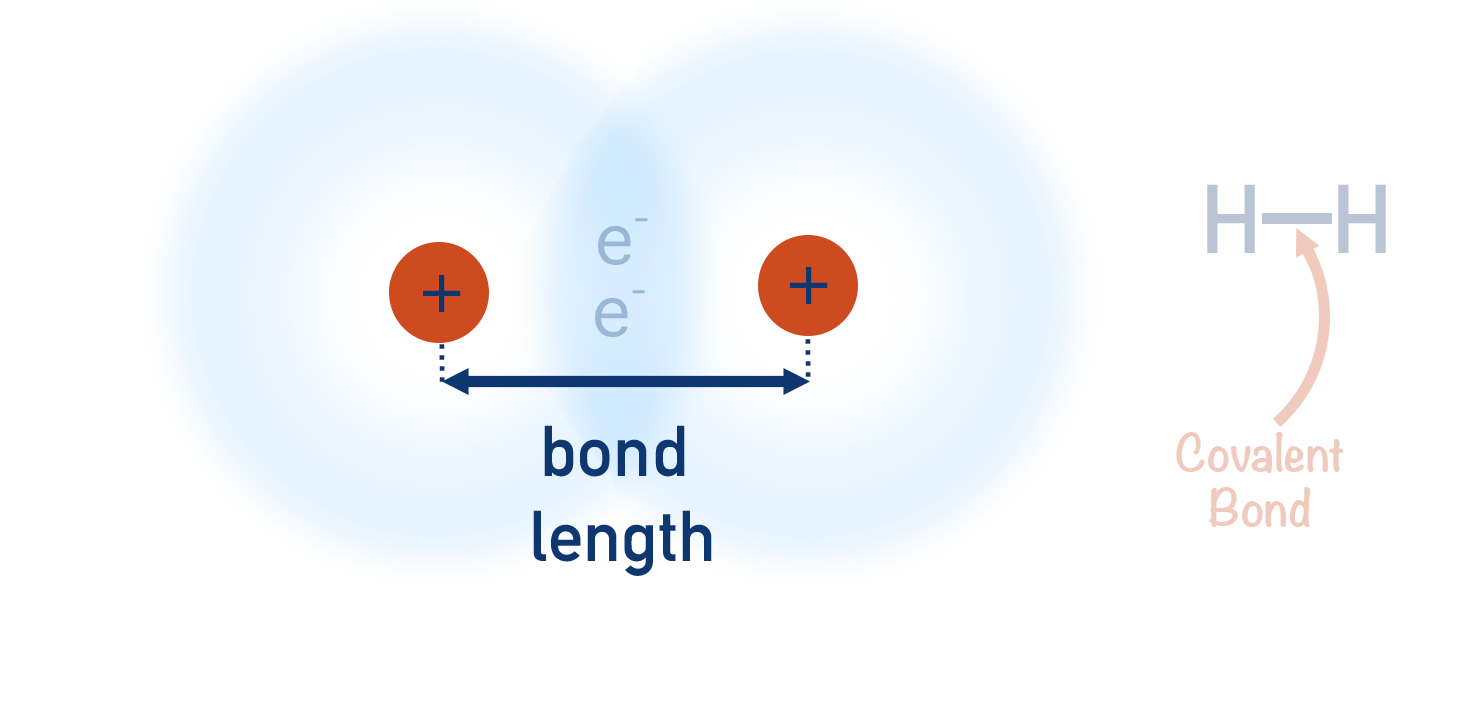
- A covalent bond is the sharing of one pair of electrons between two atoms.
- Two atomic orbitals from two atoms overlap and create a new area of electron density between the two atoms, called a molecular orbital.
- The positively charged nuclei of both atoms get attracted to the shared pair of negatively charged electrons and this pulls them closer together.
- The distance between the centre of each nuclei to each other is called a ‘bond length’.
- Non-metals form covalent bonds with other non-metals.
- As covalent bonds occur between atoms, they are atomic bonds.
Electronegativity
- Electronegativity is the ability or 'tendency' of an atom to attract a shared pair of electrons in a covalent bond towards itself.
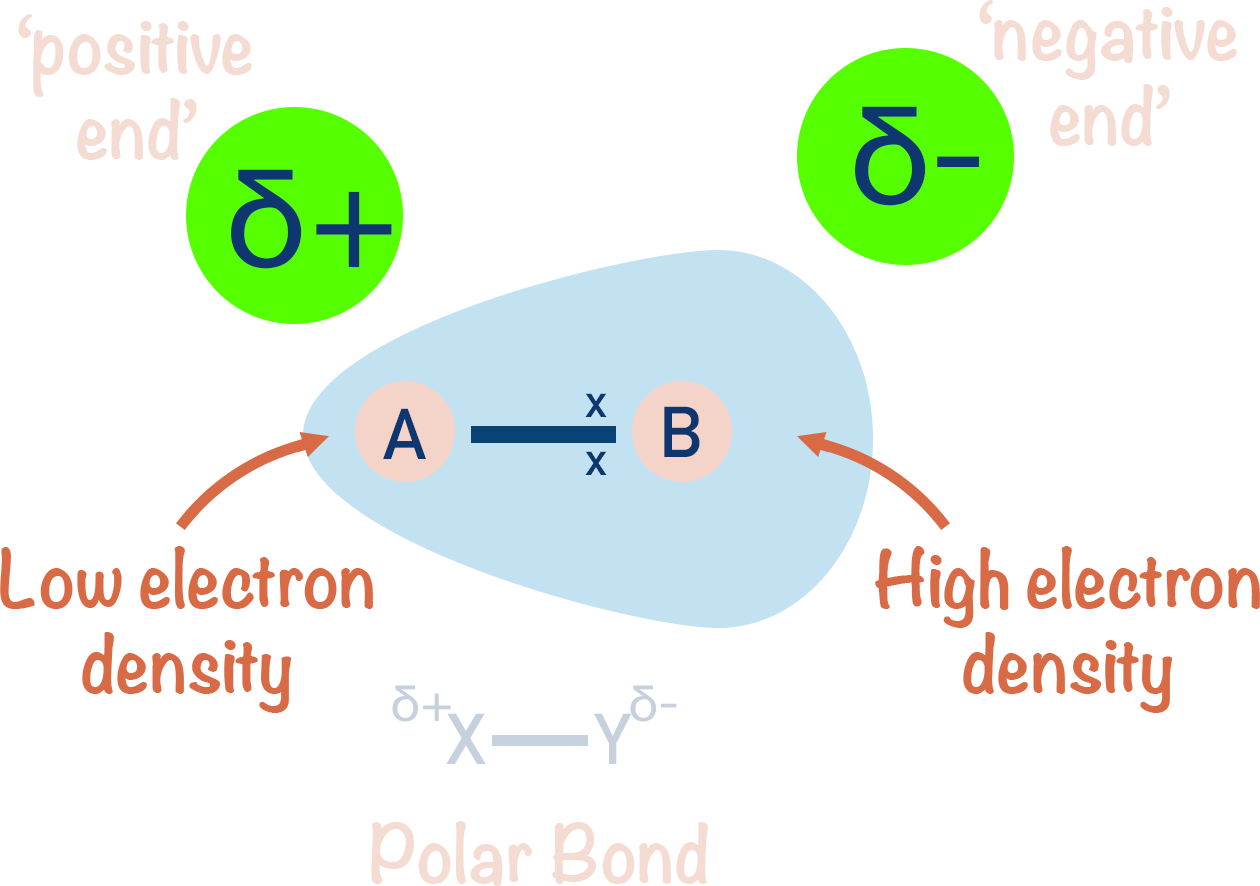
- If two atoms with different electronegativities are covalently bonded together, the bond will be polar.
- The atom with the higher electronegativity will attract the bonded electrons towards itself and will end up with a partial negative charge (δ-).
- The atom with the lower electronegativity will have the bonded electrons pulled away from it and will end up with a partial positive charge (δ+).
- The greater the difference in electronegativity between two bonded atoms, the more polar the bond.
- Electronegativity increases across a period in the periodic table and decreases down a group.
- Electronegativities are relative and the Pauling Scale is often used to compare the electronegativities of different elements - Francium has the lowest electronegativity (0.7) and Fluourine the highest (4.0).
Ionic Bonding
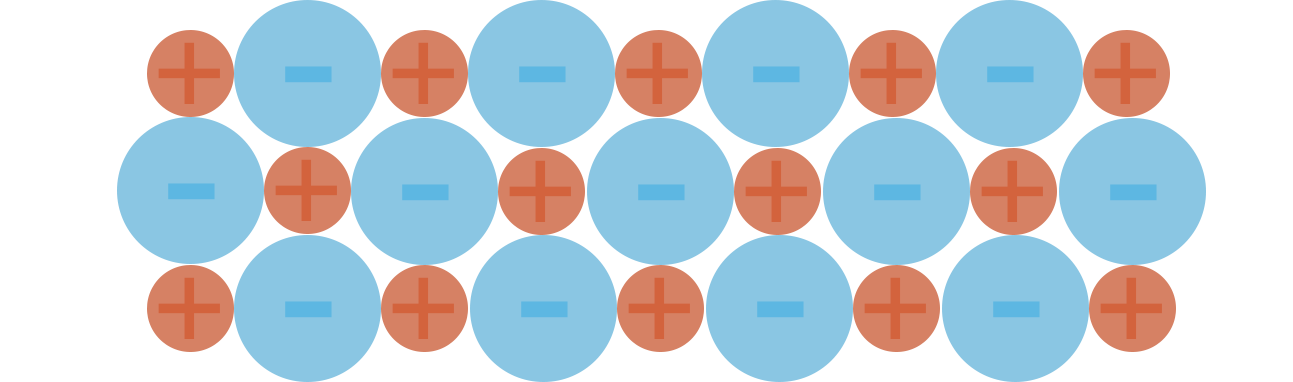
- Ionic bonding describes the electrostatic attraction that holds oppositely charged ions together.
- The strength of an ionic bond is based on the charge and size of the ions involved:
- Greater the charge = stronger the bond.
- Smaller the ionic radius = stronger the bond
- To get a stable electron configuration, atoms lose or gain electrons.
- Metals lose electrons to get a full outer shell and become positively charged ions.
- Non-metals (usually) gain electrons to get a full outer shell and become negatively charged ions.
- Oppositely charged ions arrange themselves in a solid to maximise attraction of opposite charges and minimise repulsion to like charges, forming a repeating lattice structure.
Metallic Bonding
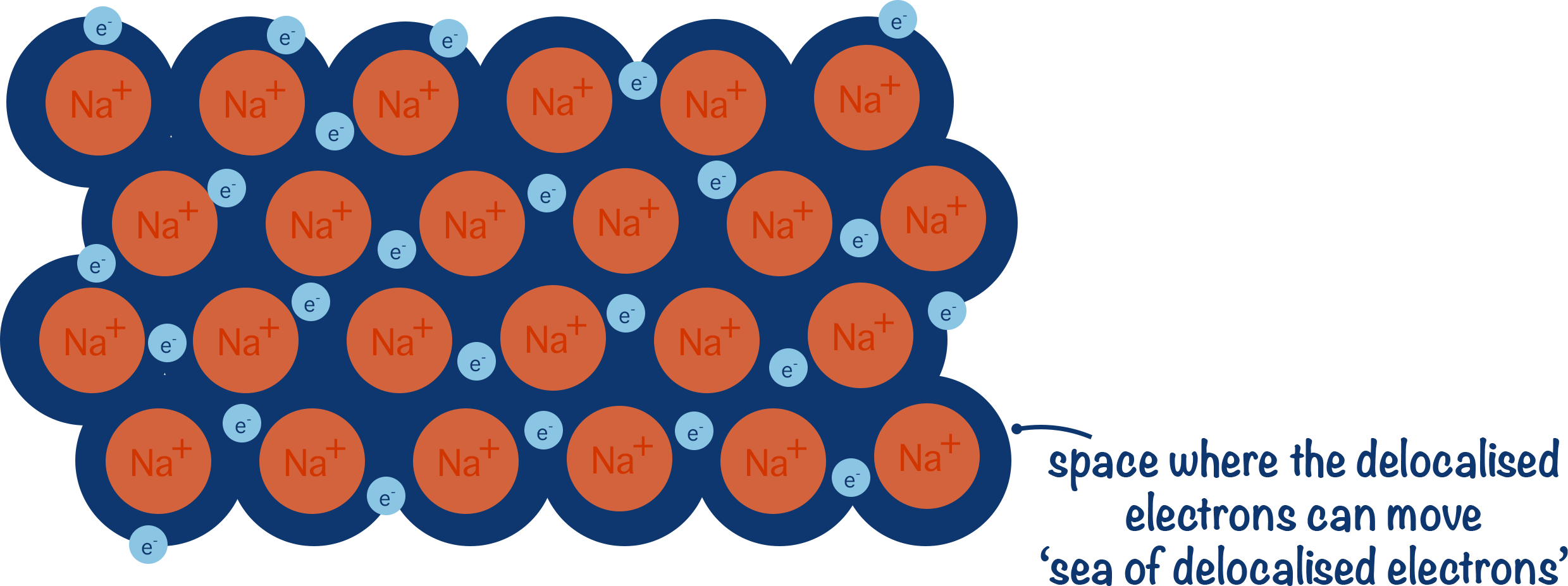
- In metallic bonding, positive metal ions are attracted to negatively charged delocalised electrons, forming a dense and rigid structure.
- Positively charged metal ions form because outermost electrons are held weakly by the metal atoms nucleus and are able to drift out of their orbitals, becoming delocalised and leaving a positively charged metal ion behind.
- Delocalised electrons from metal atoms have 'nowhere to go' and stay attracted to the positive charge of metal ions.
- Electrons are not held by the metal atom but are free to move – they are delocalised.
- Free movement of delocalised electrons enable metals to conduct electricity.
Intermolecular Forces
- Intermolecular forces hold molecules together in molecular substances.
- Temporary induced dipole-dipole forces (London dispersion forces) exist between all molecules.
- Unequal electron distribution around a molecule creates an instantaneous dipole that can induce a dipole on a neighbouring molecule.
- Two opposite dipoles from different molecules are attracted to each other and a weak force occurs.
- They are the weakest type of intermolecular force.
- The bigger the molecules, the greater the intermolecular forces that can arise between them, giving larger molecules higher melting and boiling points.
- Partial charges are shown using δ+ (partially positive) and δ- (partially negative).
Permanent Dipole-Dipole
- Permanent dipole-dipole interactions exist between polar molecules.
- The partially negatively charged area of one molecule is attracted to a partially positively charged area of another molecule.
- The attractions are permanent and are stronger than temporary, induced dipole-dipole forces.
- The greater the polarity of a bond or molecule, the stronger the permanent dipole-dipole interactions.
Hydrogen Bonding
- Hydrogen bonds are a specific type of intermolecular force.
- Molecules that can form hydrogen bonds have higher melting and boiling points than similiar sized molecules that are unalbe to.
- Hydrogen bonds exist between molecules that have a N-H, O-H or F-H bond.
- Nitrogen, oxygen and fluorine are the most electronegative elements, so the covalent bonds between them and hydrogen are highly polar.
- Highly polar bonds result in strong attractive forces between molecules.
- The partially negative nitrogen, oxygen or fluorine forms hydrogen bonds with the partially positive hydrogen atoms from other molecules.
- Substances that can form hydrogen bonds have higher melting points than similar-sized substances that cannot.
Energy and Enthalpy
Enthalpy Changes
- During chemical reactions energy is exchanged between reacting substances and their surroundings.
- Exothermic reactions – products are lower in energy than the reactants and the temperature of the surroundings increases.
- Endothermic reactions – products are higher in energy than the reactants and the temperature of the surroundings decreases.
- Enthalpy is a measure of heat energy with the units kJmol-1.
- The actual amount of energy in a compound or atom is not easily measured in chemistry, so it is the change in heat energy during a reaction (enthalpy change, ΔH) that is measured.
- Exothermic reactions have negative enthalpy changes (-ΔH).
- Endothermic reactions have positive enthalpy changes (+ΔH).
- Standard enthalpy change refers to the thermal energy change that occurs during a reaction, where 1 mole of product is formed in standard conditions (101 kPa and 298K).
Bond Enthalpies
- The overall enthalpy change that occurs in a reaction can be found by subtracting the sum of all the bond enthalpies of bonds formed from the sum of all bond enthalpies of bonds broken.
- A bond enthalpy is the amount of energy released or absorbed when a particular bond is made or broken.
- Energy is released by two atoms when a bond is formed between them.
- That same amount of energy is absorbed by the two atoms when the bond is broken.
- Mean bond enthalpies are used to account for the different environments a bond might be in.
Calorimetry
- Calorimetry is a technique used to find the heat energy change that occurs during a reaction.
- Heat energy change is found by measuring how the temperature of the substance surrounding the reaction changes.
- Specific heat capacity (c) refers to the energy required to raise the temperature of 1g (or 1kg) of a substance by 1oC.
- The temperature change of a substance can be used with its specific heat capacity to find the energy it has absorbed or released.
- Change in energy = mass x specific heat capacity x change in temperature
- Q = mcΔT
- Change in energy = mass x specific heat capacity x change in temperature
- Energy is often lost from the substance, whose temperature is being measured, to the surroundings.
- To increase the accuracy of calorimetry, heat loss must be minimised (extra insulation can help).
Hess' Law
- Hess’ Law states that the energy change that occurs when a substance is changed into a product is the same, regardless of the route taken.
- There is often more than one way to go from a reactant to a product, meaning there is more than one ‘route’ to convert a substance to a particular product.
- Hess cycles are ways of drawing out the energy changes that occur when a substance is turned into a different substance via more than one route.
Kinetics
Collision Theory
- Kinetics is the study of how fast reactions occur.
- How quickly a reaction happens is called its reaction rate.
- Reactions occur when reactant particles collide together with enough energy (activation energy, Ea).
- Collisions between particles that lead to a reaction are called ‘successful collisions’.
- Increasing the frequency of successful collisions increases the rate of reaction.
- Increasing the temperature, concentration and pressure of reacting particles increases the rate of a reaction.
Boltzmann Distribution Curves
- Maxwell-Boltzmann distribution curves show how available energy is distributed among molecules in a gaseous system.
- At a given temperature, only a certain amount of energy is available for all molecules of gas within a system.
- Individual molecules have different energies and are constantly colliding, transferring energy with other molecules – either gaining or losing energy.
- The effects of changing concentration, temperature and the use of a catalyst on the energies of particles can be shown.
Equilibrium
- In a reversible reaction, reactants react to produce products (forward reaction) and those products also react to produce the original reactants (reverse reaction).
- At equilibrium, the rates of both the forward and reverse reactions are the same – the concentrations of all reactants and products do not change.
- The position of equilibrium describes how much one reaction has been favoured compared to the other before equilibrium is reached.
- Changing the conditions of an equilibrium system can cause the position of equilibrium to move.
- Le Chatelier’s Principle states that the position of equilibrium will move to oppose a change in conditions.
- Increasing the temperature of a system forces the position of equilibrium to move to favour the endothermic reaction.
- Increasing the concentration of a reactant forces the position of equilibrium to move to favour the reaction that uses up that reactant.
- Increasing the pressure of a system forces the position of equilibrium to move to favour the reaction that produces the fewest moles of gas.
