Video Tutorial Amides
Quick Notes Amides
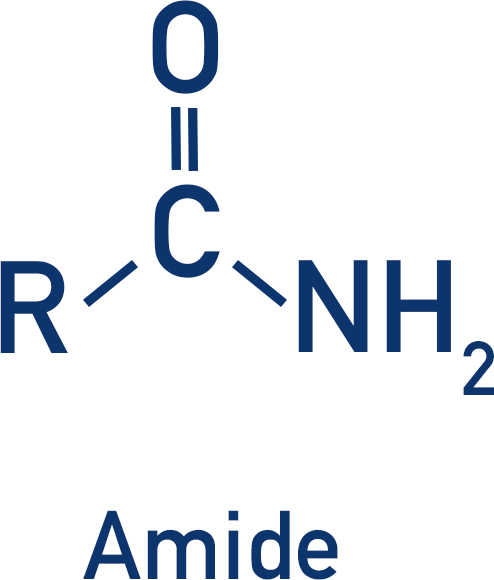
- Amides are carboxylic acid derivatives that have a carbonyl group with an amine attached to the carbon from the carbonyl. They are generally unreactive.
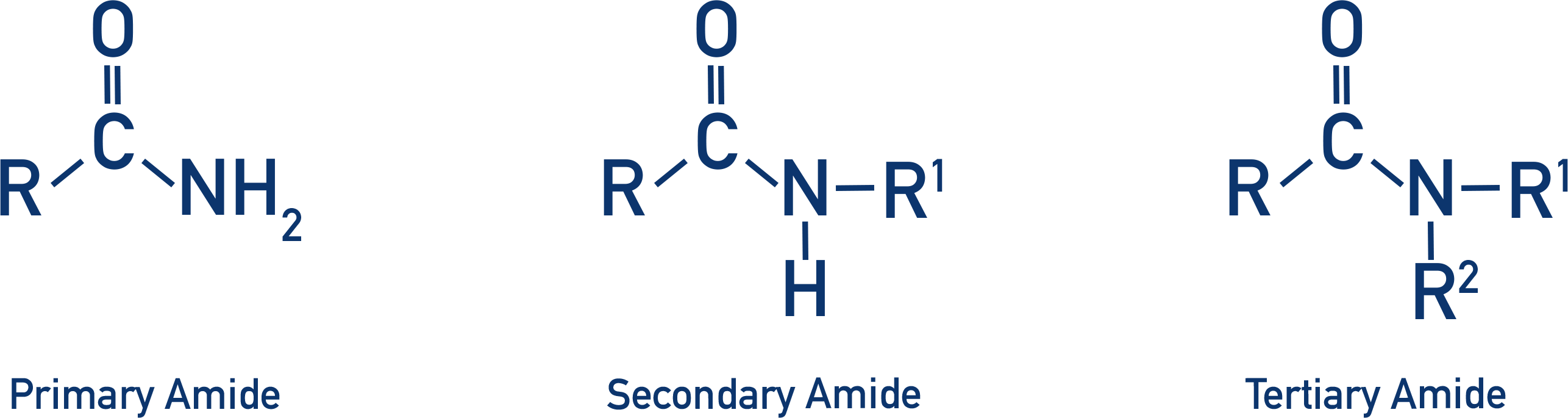
- Amides can be primary, secondary and tertiary.
- (Primary) amides are formed from acyl chlorides and ammonia in a nucleophilic addition-elimination reaction, producing an ammonium chloride salt.
- Secondary amides are formed from acyl chlorides and a primary amine.
- Tertiary amides are formed from acyl chlorides and a secondary amine.
Full Notes Amides
Amides are derivatives of carboxylic acids, they have a carbonyl bond and an amine group attached to the same carbon. Amides are generally unreactive.
Amides can be primary, secondary or tertiary. Primary amides have a nitrogen atom bonded to only one carbon atom, secondary amides have a nitrogen atom bonded to two carbon atoms and tertiary amides have a nitrogen atom bonded to three carbon atoms.

Preparation of Primary Amides
Even though amides are carboxylic acid derivatives (‘come from’ carboxylic acids) it’s very difficult to prepare amides directly from carboxylic acids. This is because ammonia is used to form an amide. Ammonia is a strong base, so as soon as it comes into contact with a carboxylic acid it just accepts a proton from the acid, producing a carboxylate ion and ammonium ion.
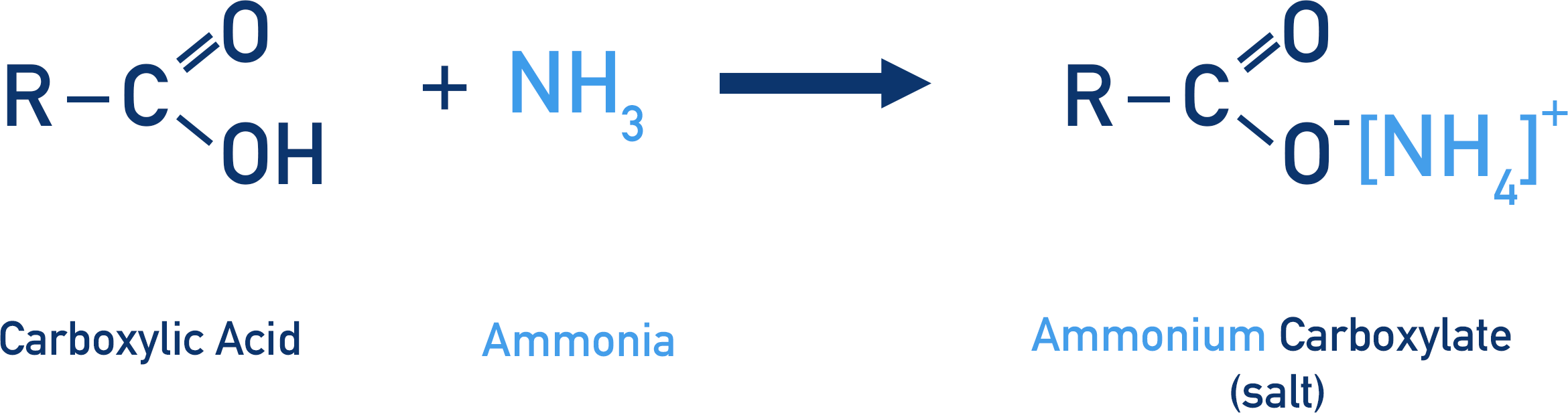
To form a bond between the amine group and the carbon in the acyl group, the ammonia must act as a nucleophile.
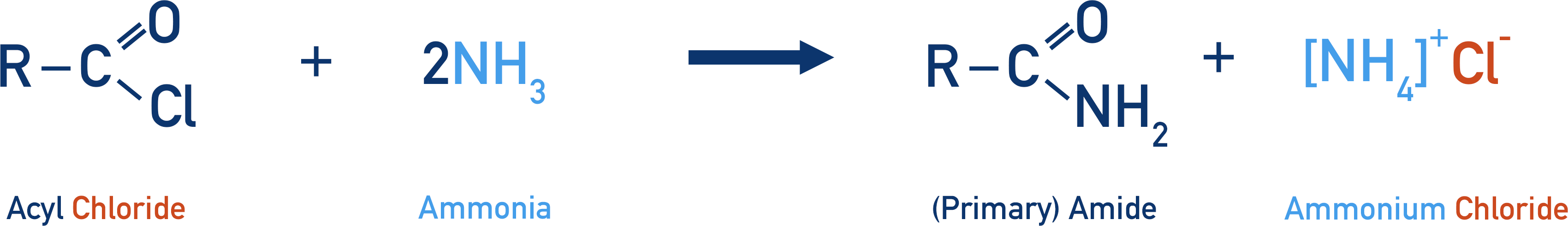
Acyl chlorides are a common starting reactant as the carbon in the acyl group is highly electron deficient, so it attracts the lone pair of electrons in the nitrogen atom from the ammonia (which behaves as a nucleophile).
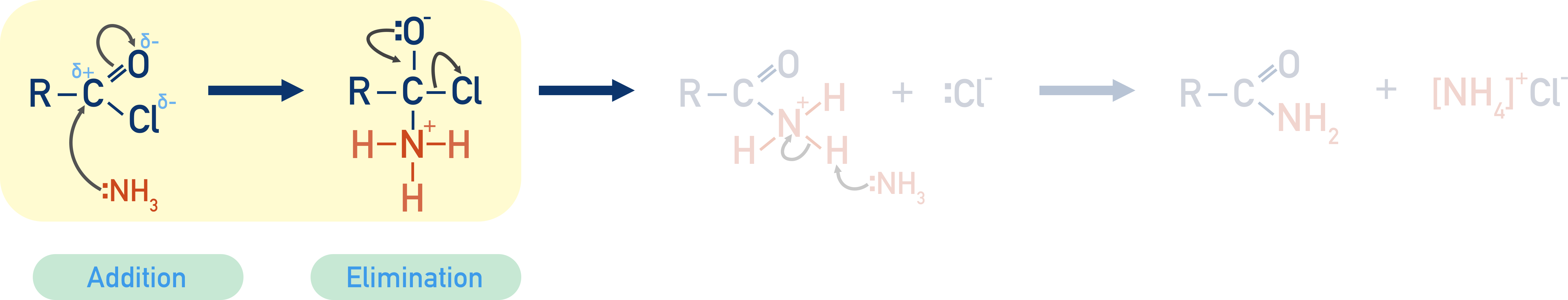
The nucleophile (ammonia) is added to the acyl chloride, then a chloride ion is eliminated and removed. This reaction is a nucleophilic addition-elimination reaction.
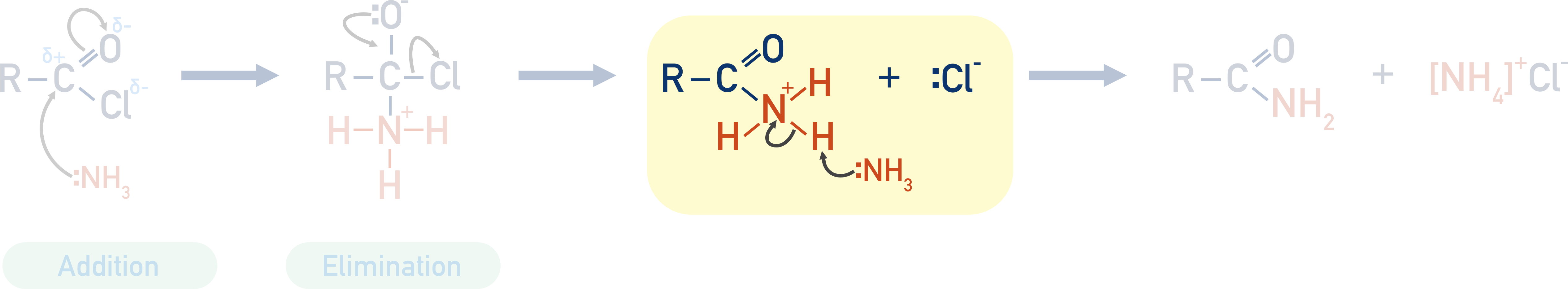
The positive hydrogen ion released (by the ammonia acting as a nucleophile) is picked up by another ammonia molecule, and an ammonium ion is formed. This positively charged ammonium ion is attracted to the negatively charged chloride ion, producing ammonium chloride.
Preparation of Secondary and Tertiary Amides
If a primary amine is used (instead of ammonia) as a nucleophile, the amide formed is a secondary amide and hydrochloric acid is produced (there is no ammonia to ‘pick-up’ the positive hydrogen ion released, only the chloride ion).
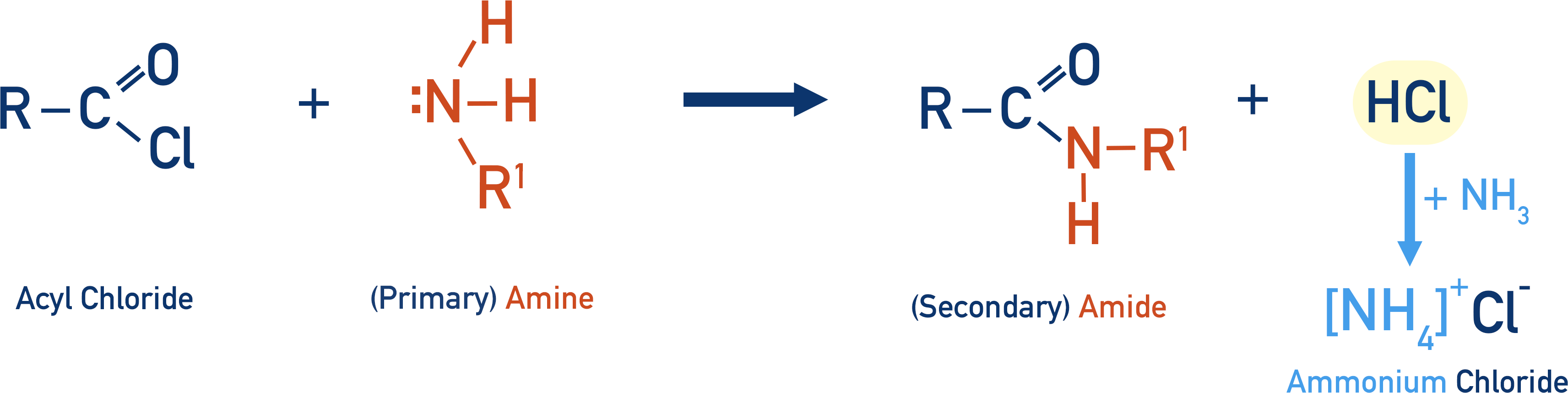
As amides are generally unreactive, ammonia can now be added to remove the HCl as ammonium chloride salt.
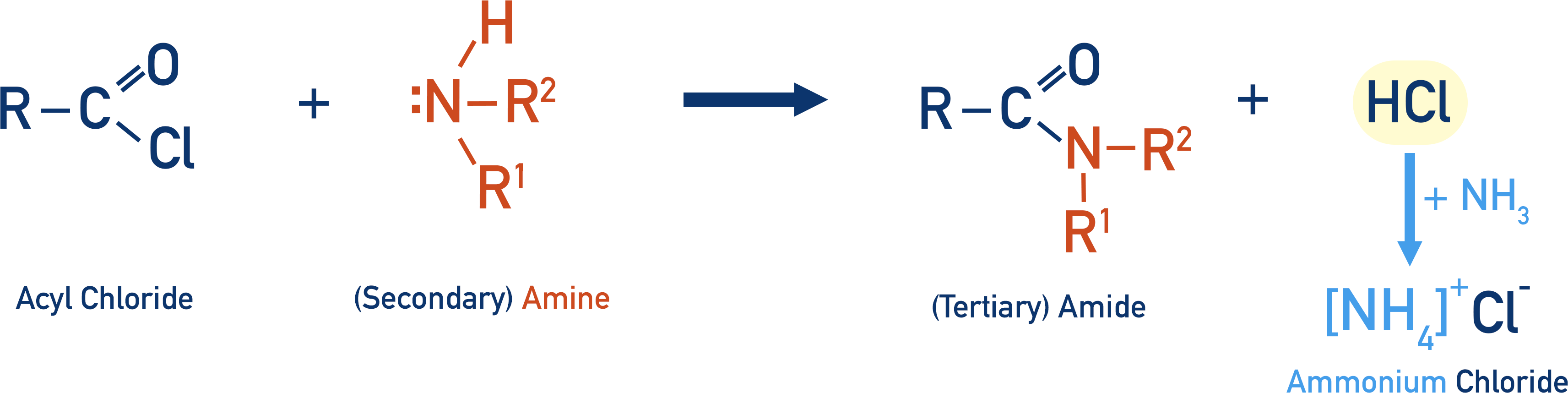
If a secondary amine is used instead of ammonia, the amide formed is a tertiary amide.
We’ve launched our new site! 🎉
Course-specific notes with built-in search!
AP • A-Level (AQA • CIE • Edexcel • OCR) • IB • NCERT 11 + 12
over 750+ new pages and 3,500 images.
Visit the new homepage
