Electrode Potentials and Cells
Quick Notes: Electrode Potentials and Cells
- A simple half cell consists of a metal electrode in contact with an electrolyte that contains its ions.
- The electrode in a half cell has an electrical potential, based on the position of equilibrium for the reduction reaction of ions at the electrode surface.
- Electrochemical cells can be used to measure the relative potentials of different electrodes.
- Electrochemical cells are made up of two different half cells connected together, with a salt bridge between the electrolytes from each.
- Standard electrode potential (E°) is the potential of a half-cell compared to the Standard Hydrogen Electrode (SHE).
- Standard conditions for E° measurements: 298 K; 1.00 mol dm⁻³ ion concentration; 100 kPa pressure for gases.
- Cell potential (E°cell) = E°(cathode) − E°(anode).
- Standard electrode potentials can be listed as an electrochemical series, with more positive E° values indicating stronger oxidising agents.
- Conventional representation of cells: | separates phases, || represents the salt bridge, oxidation half cell on left, reduction half cell on right.
Full Notes: Electrode Potentials and Cells
Electrochemistry and Electrode Potentials are covered in more detail
here.
This page is just what you need to know for AQA A-level Chemistry :)
What Are Electrode Potentials?
Every chemical species has a tendency to either gain or lose electrons. We can measure this tendency using something called electrode potentials, E°. Electrode potentials tell us how easily a species can be reduced (gain electrons) or oxidised (lose electrons).
- A more positive electrode potential means the species is more likely to gain electrons (be reduced).
- A more negative electrode potential means the species is more likely to lose electrons (be oxidised).
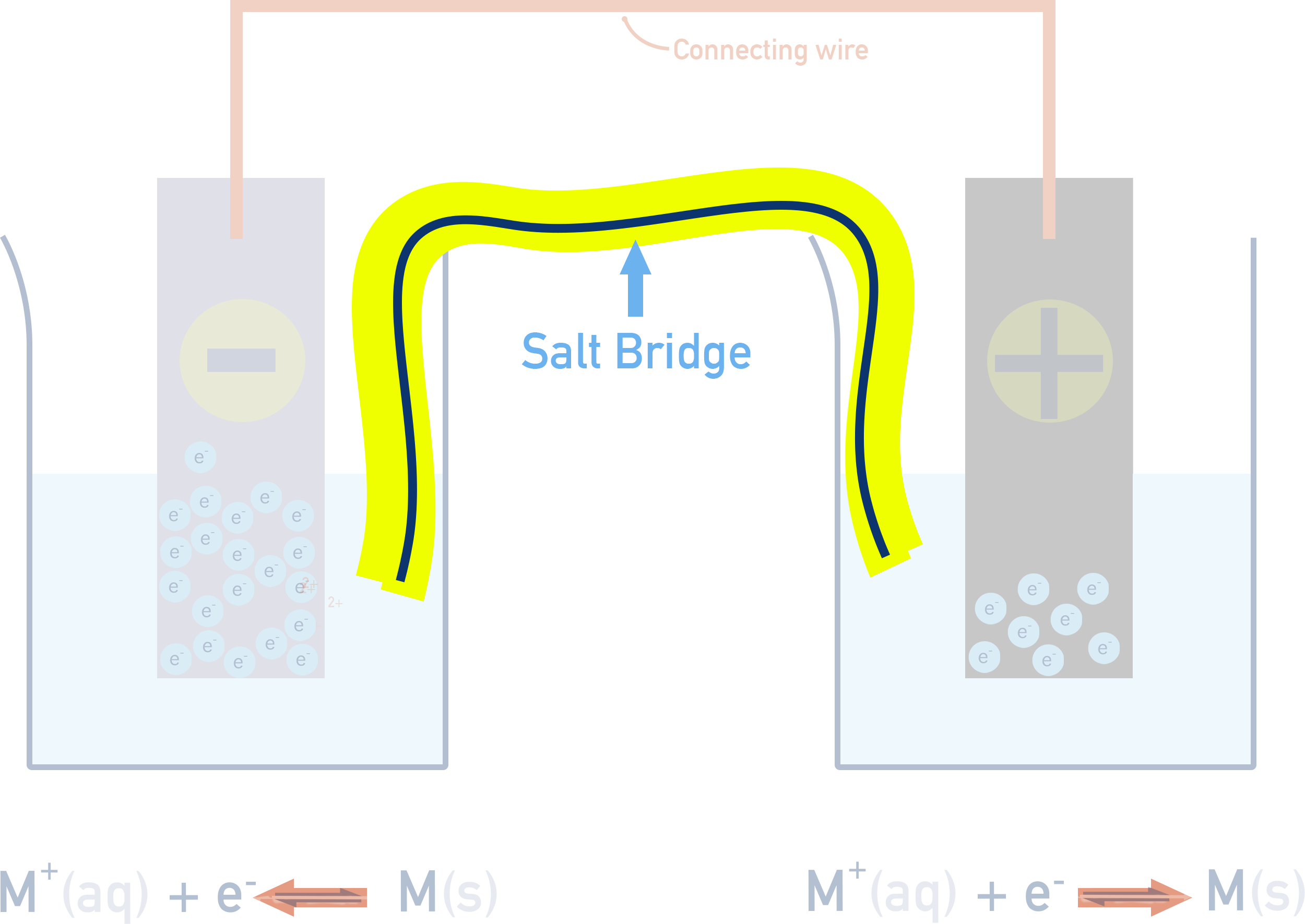
To measure electrode potentials, electrochemical cells are used. Electrochemical cells are made up of two different half-cells connected by a salt bridge.
Half-Cells
Simple half-cells are made of a metal solid placed into a solution that contains ions of the metal. The metal solid is called an electrode and the solution it is in an electrolyte.
A redox equilibrium is established between the ions in the electrolyte and the solid metal electrode.
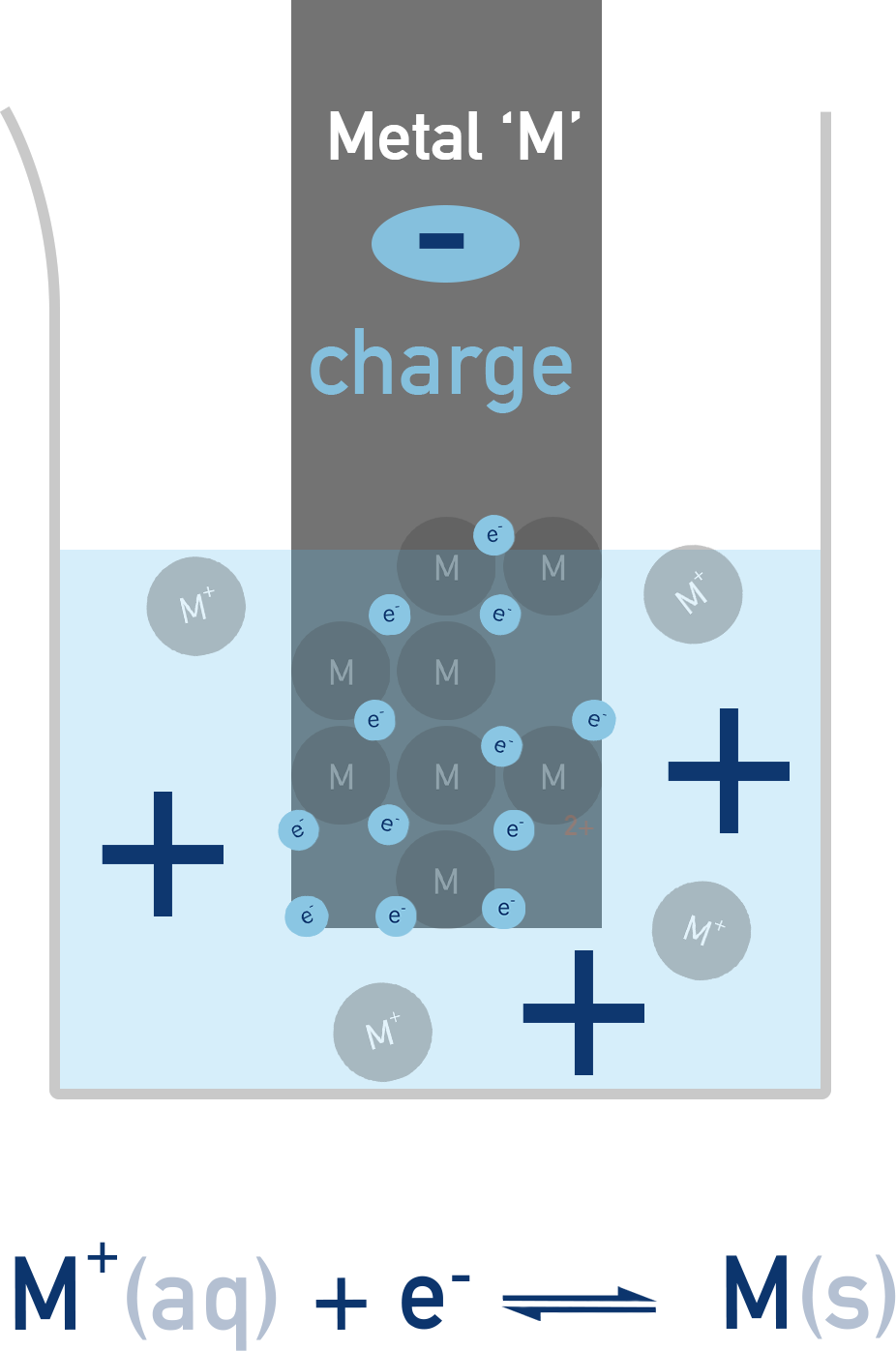
This sets up a potential difference between the metal and the solution, that depends on how far the equilibrium lies to the left or right. This potential difference is the electrode potential.
The electrode potential can’t be measured directly however we can compare different electrode potentials for different half-cells by connecting them to a reference half-cell and measuring the potential difference each time.
The reference used is the Standard Hydrogen Electrode (SHE).
The Standard Hydrogen Electrode (SHE)
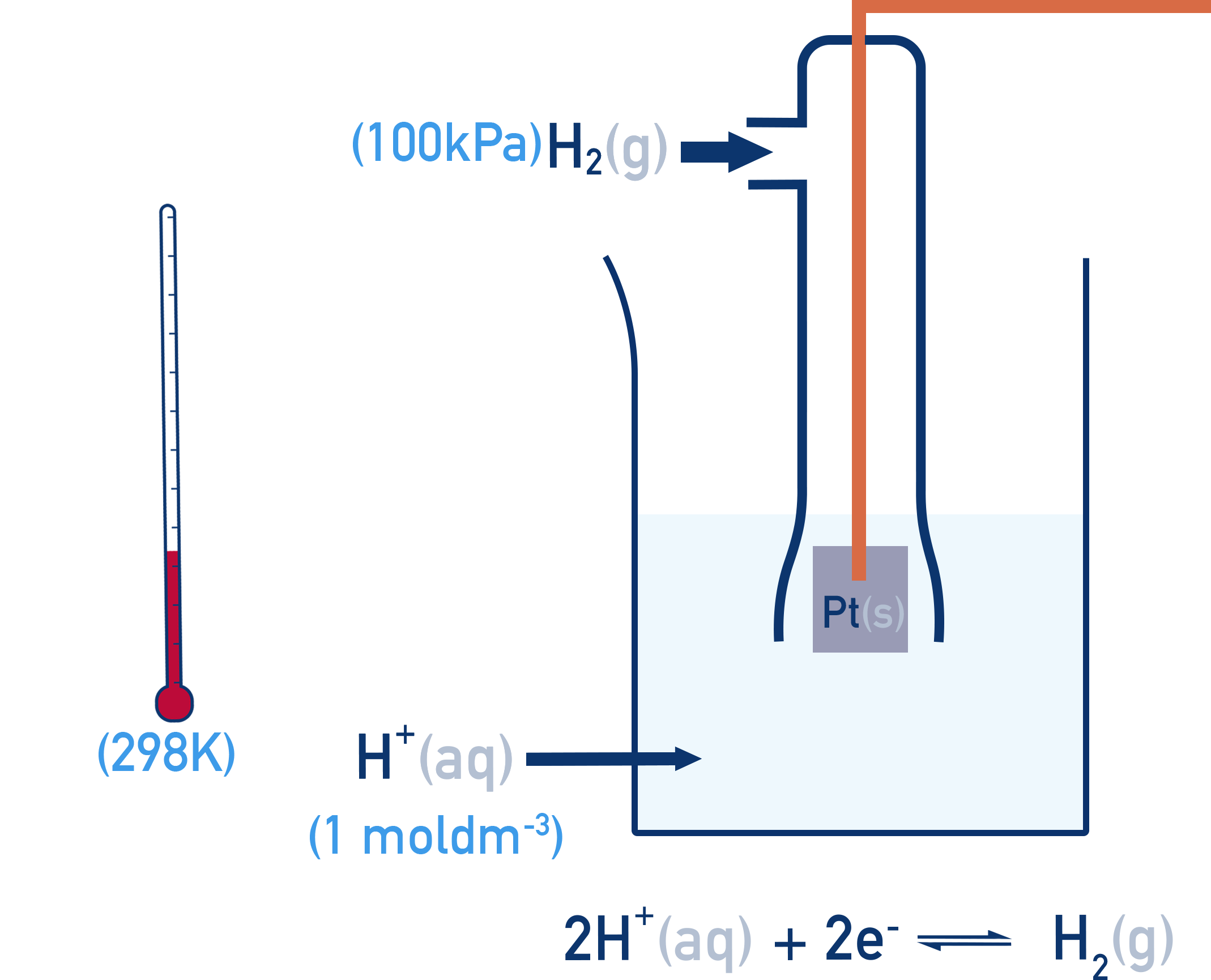
- Hydrogen gas at 100 kPa
- Bubbled over a platinum electrode
- In 1.00 mol dm⁻³ H⁺ solution
- At 298 K (25°C)
All standard electrode potentials (E°) are measured under these conditions and describe the potential of a half-cell compared to the SHE. The Standard Hydrogen Electrode (SHE) is assigned a potential of 0.00 V. All this means is that when two standard hydrogen electrodes are connected together, the potential difference is 0.00 V.
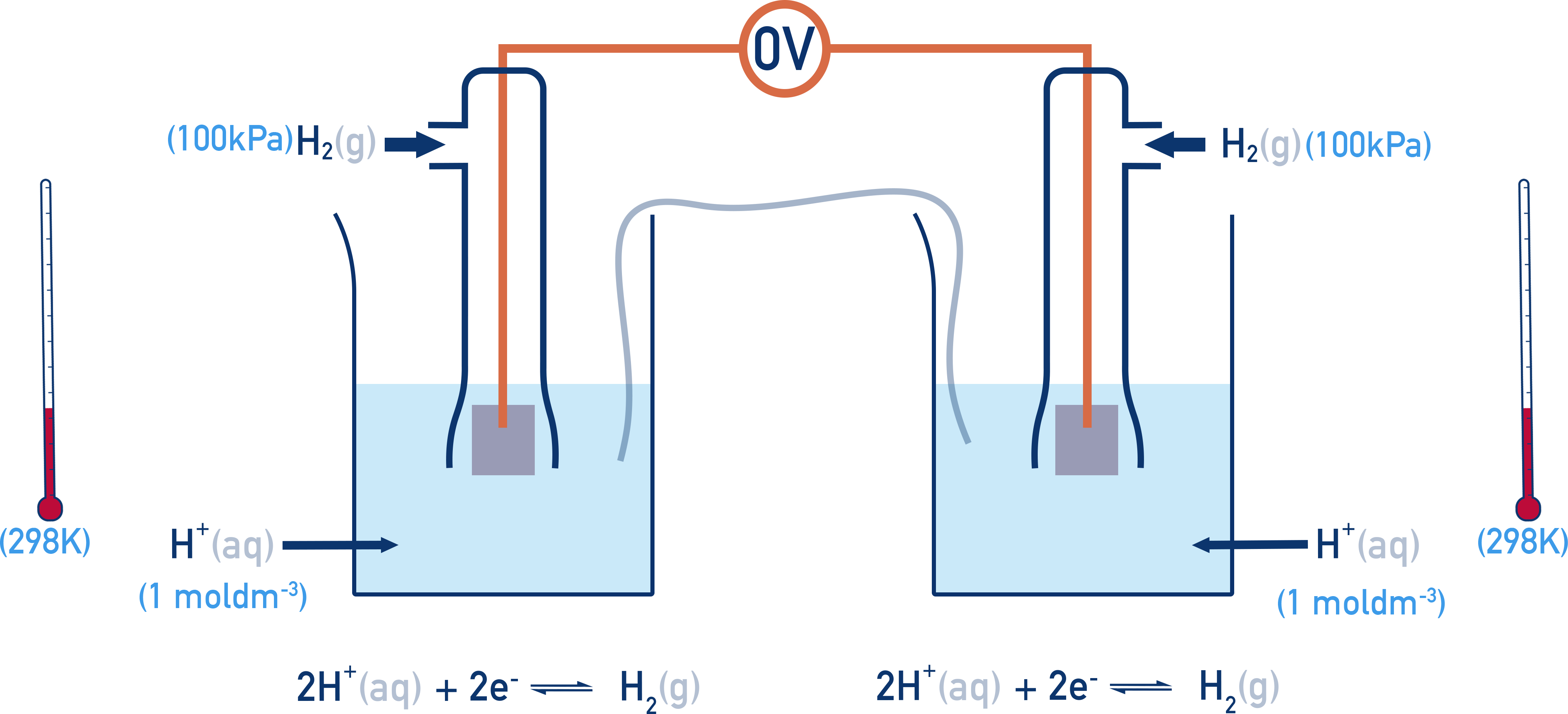
If the right-hand half cell is now changed, a potential difference (voltage) is measured.
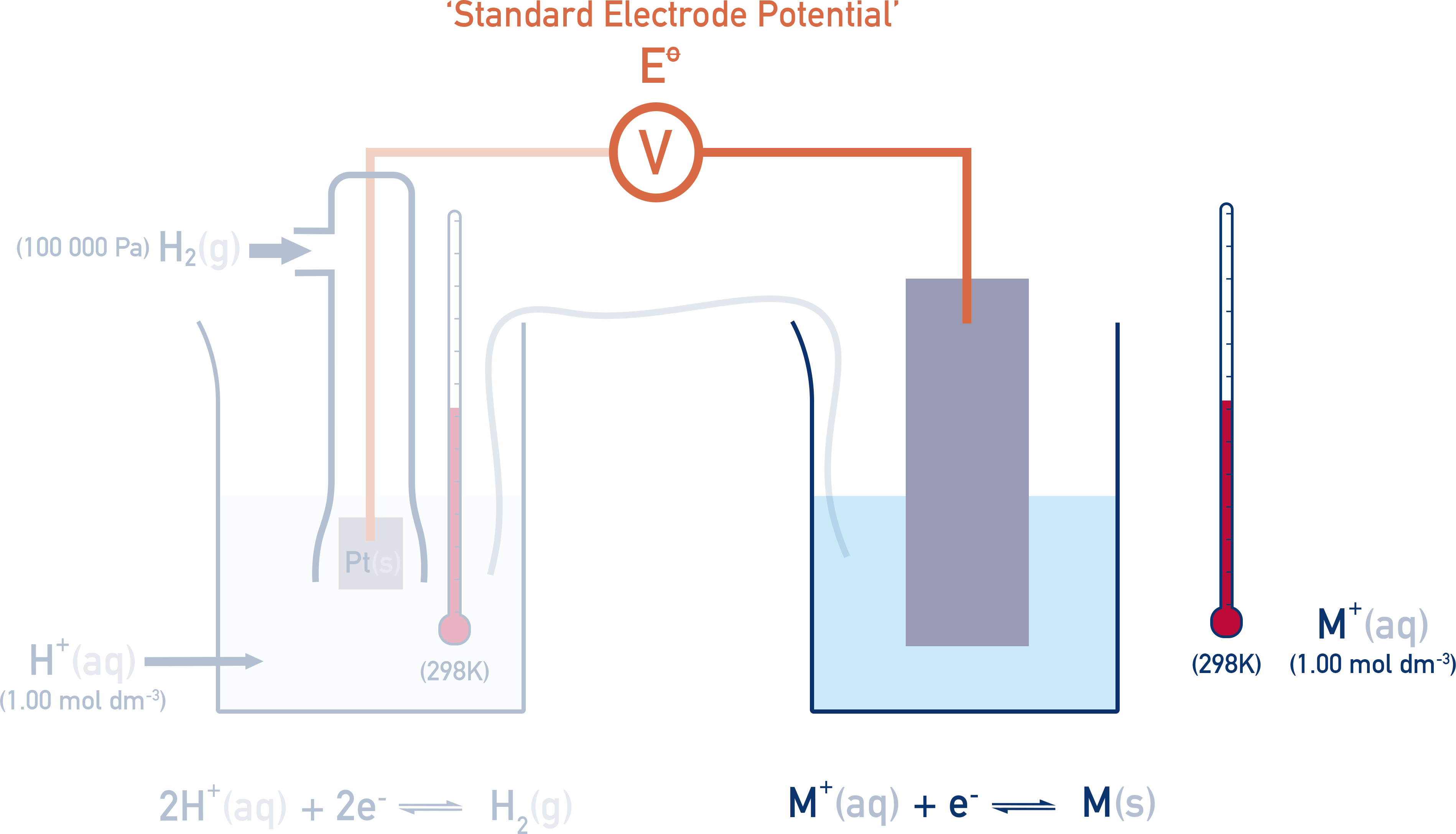
This measured value is called the standard electrode potential (E° value) of the right-hand half cell.
The temperature, concentration and pressure (for gases) must be the same as the standard hydrogen electrode (1 mol dm⁻³, 298 K and 100 kPa of pressure), otherwise positions of equilibrium in each half cell will be affected and comparisons between measured potentials won’t be representative.
Standard electrode potentials are often put into a table called the electrochemical series.
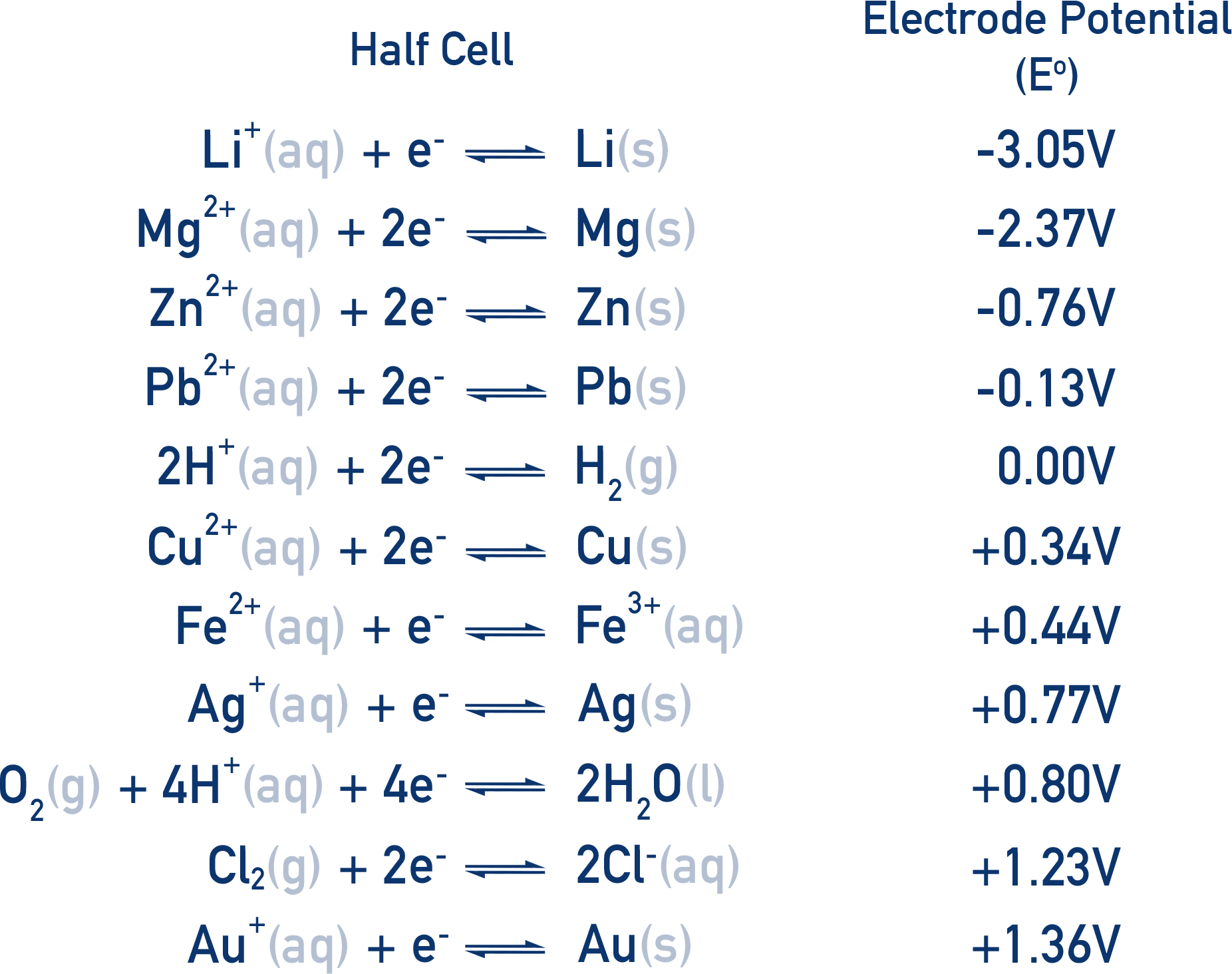
The more positive the E°, the more likely the species in the half cell is to be reduced. The more negative the E°, the more likely the species in the half cell is to be oxidised.
For exampleIf we compare the following two standard electrode potentials:
Zn²⁺(aq) + 2e⁻ ⇌ Zn(s) E° = −0.76 V
Cu²⁺(aq) + 2e⁻ ⇌ Cu(s) E° = +0.34 V
The more positive value of Cu²⁺/Cu tells us that Cu²⁺ is better at gaining electrons and being reduced than Zn²⁺. Cu²⁺ is a stronger oxidising agent than Zn.
Equally, the Zn(s) is more likely to be oxidised than the Cu(s). Meaning the Zn(s) is a stronger reducing agent than Cu(s).

Remember the redox equilibria written for standard electrode potentials are written in the forward direction (reduction). This means the reverse direction is oxidation. More positive E° values mean forward direction is more likely (reduction), more negative E° values mean reverse direction is more likely (oxidation).
Building an Electrochemical Cell
When two different half-cells are connected together by a wire, electrons will flow through the wire from the half-cell with the more negative electrode potential (stronger reducing agent) to the one with the more positive electrode potential (stronger oxidising agent).
- The half-cell where oxidation happens is called the anode (electrons lost).
- The half-cell where reduction happens is the cathode (electrons gained).
- The salt bridge completes the circuit by allowing ions to move and balance charge.
Standard Cell Potential (E°cell)
The overall voltage (cell potential) produced by an electrochemical cell is the difference in potential between the two half-cells.
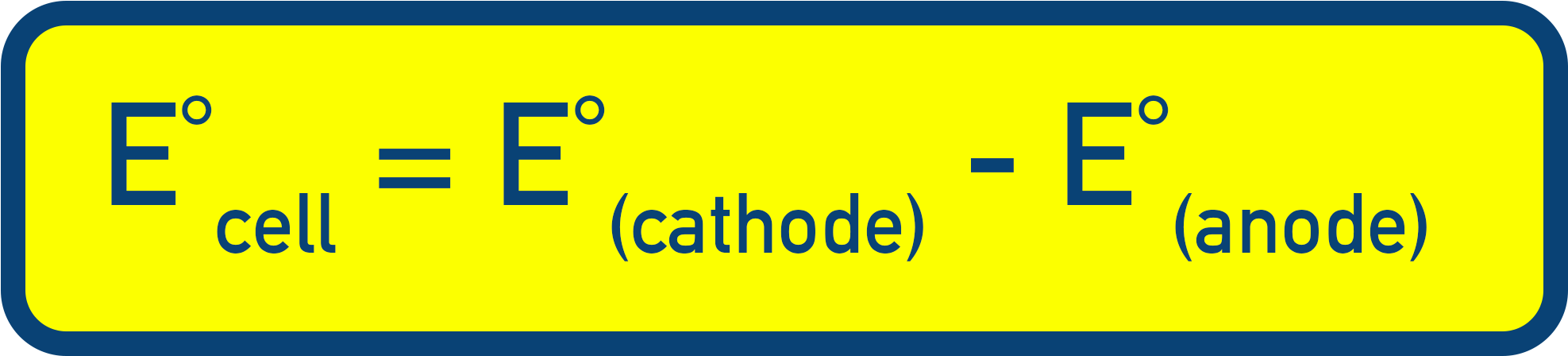
- E°cathode is the more positive electrode (reduction).
- E°anode is the more negative electrode (oxidation).
This value tells us the driving force of the redox reaction — a bigger E°cell means a more spontaneous reaction (see Gibbs Free Energy Change).
The following two half-cells are connected together. Determine E°cell:
Zn²⁺(aq) + 2e⁻ ⇌ Zn(s) E° = −0.76 V
Cu²⁺(aq) + 2e⁻ ⇌ Cu(s) E° = +0.34 V
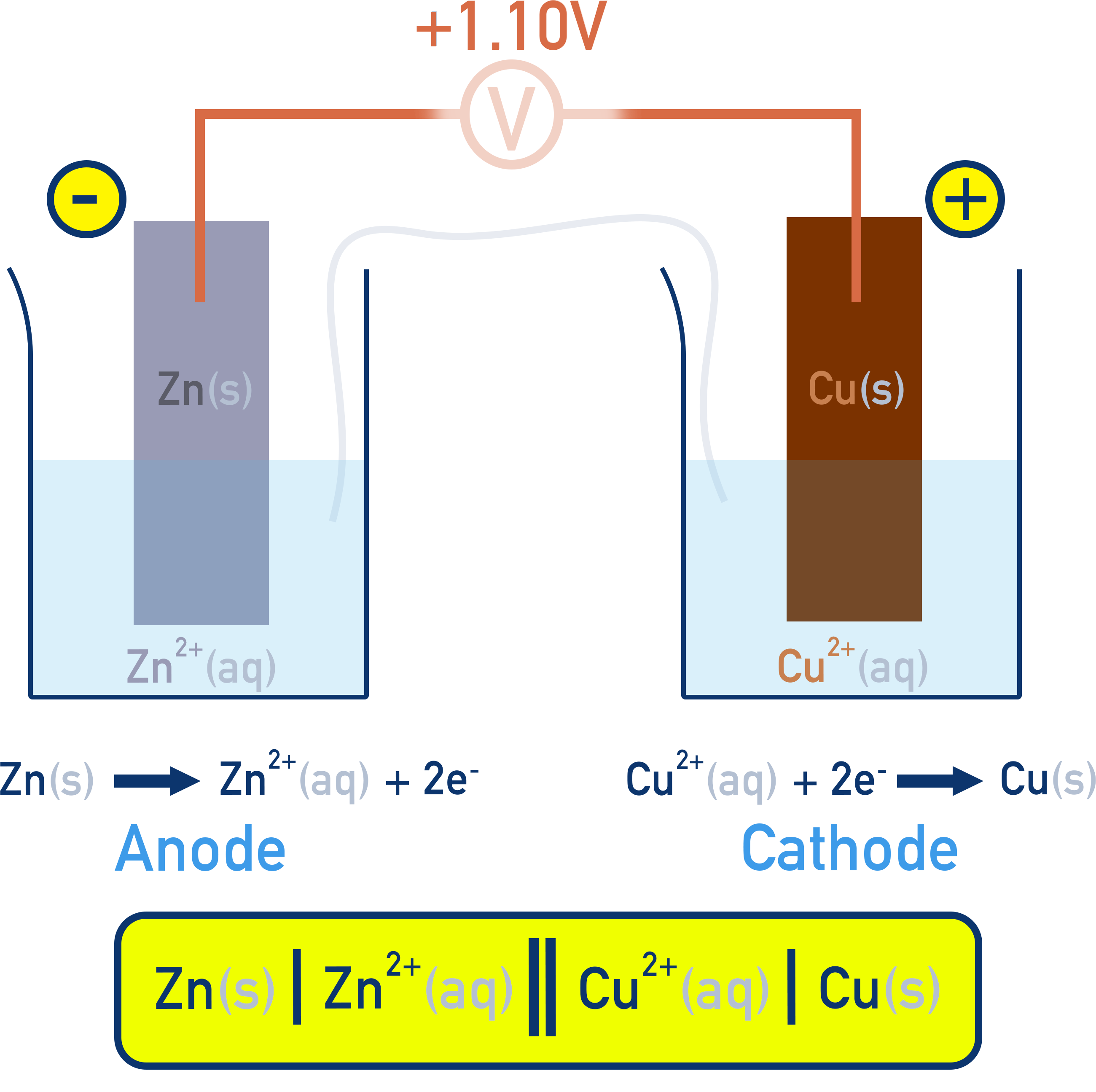
The more positive E° is for Cu²⁺/Cu (+0.34 V). This means the Cu²⁺/Cu will be the half cell where reduction occurs — the cathode. The Zn²⁺/Zn will be the half cell where oxidation occurs — the anode.
If E°cell = E°cathode − E°anode
then E°cell = (+0.34) − (−0.76) = +1.10 V
Comparing Oxidising and Reducing Strengths
Electrode potentials allow us to compare the oxidising and reducing ability of substances:
- A more positive E° means better at gaining electrons = stronger oxidising agent
- A more negative E° means better at losing electrons = stronger reducing agent
These values are usually arranged into an electrochemical series from most negative to most positive (see above), giving a powerful tool for predicting redox reactions.
Cell Notation
In electrochemistry, cells are written using a shorthand:
- A single vertical line (|) separates different phases (solid, liquid, aqueous).
- A double line (||) represents the salt bridge.
- The anode (oxidation) is written on the left, and the cathode (reduction) on the right.
For example Write the conventional cell notation for an electrochemical cell made from the following:
Zn²⁺(aq) + 2e⁻ ⇌ Zn(s) E° = −0.76 V
Cu²⁺(aq) + 2e⁻ ⇌ Cu(s) E° = +0.34 V
We’ve already established (see above) Cu²⁺/Cu is the cathode, where reduction happens. Cu²⁺ will be reduced to Cu. Equally, Zn²⁺/Zn is the anode, where oxidation happens, Zn will be oxidised to Zn²⁺.
Anode is written on the left with the Zn(s) and Zn²⁺(aq) separated by a vertical line as they are in different phases. Cathode is written on the right with the Cu²⁺(aq) and Cu(s) again separated by a vertical line.
Zn(s) | Zn²⁺(aq) || Cu²⁺(aq) | Cu(s)

Summary
- Electrode potentials measure the tendency of species to be reduced or oxidised.
- These are always compared to the Standard Hydrogen Electrode (SHE) under standard conditions.
- Half-cells can be combined to form electrochemical cells; electrons flow from more negative to more positive E° values.
- The difference in electrode potentials gives the cell voltage, E°cell = E°(cathode) − E°(anode).
- The electrochemical series helps predict the direction and feasibility of redox reactions.
