Video Tutorial Bromination of Benzene
Quick Notes Bromination of Benzene
- The bromination of benzene involves the electrophilic substitution of a bromine atom onto a benzene ring:
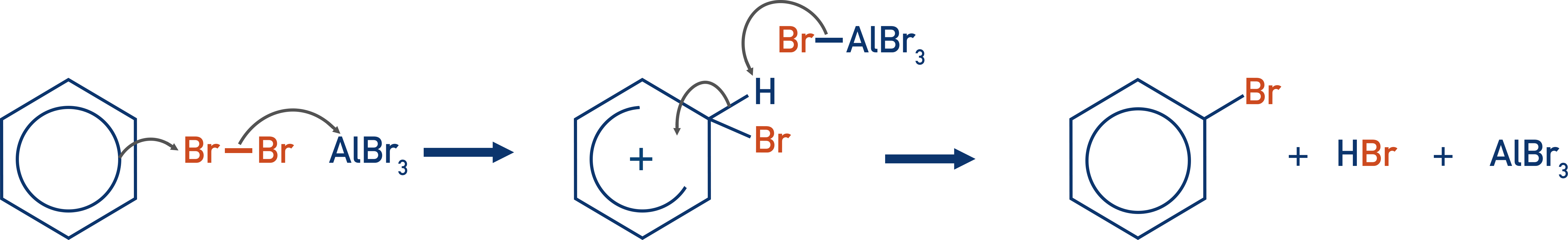
- A halogen carrier is needed (usually AlBr3) to create an electrophile, as the delocalised electrons in benzene are unable to polarise the bromine molecule sufficiently.
- Benzene reacts with bromine in electrophilic substitution reactions.
- A catalyst is needed.
- Alkenes and cyclohexene react with bromine in electrophilic addition reactions.
- No catalyst is needed.
Full Notes Bromination of Benzene
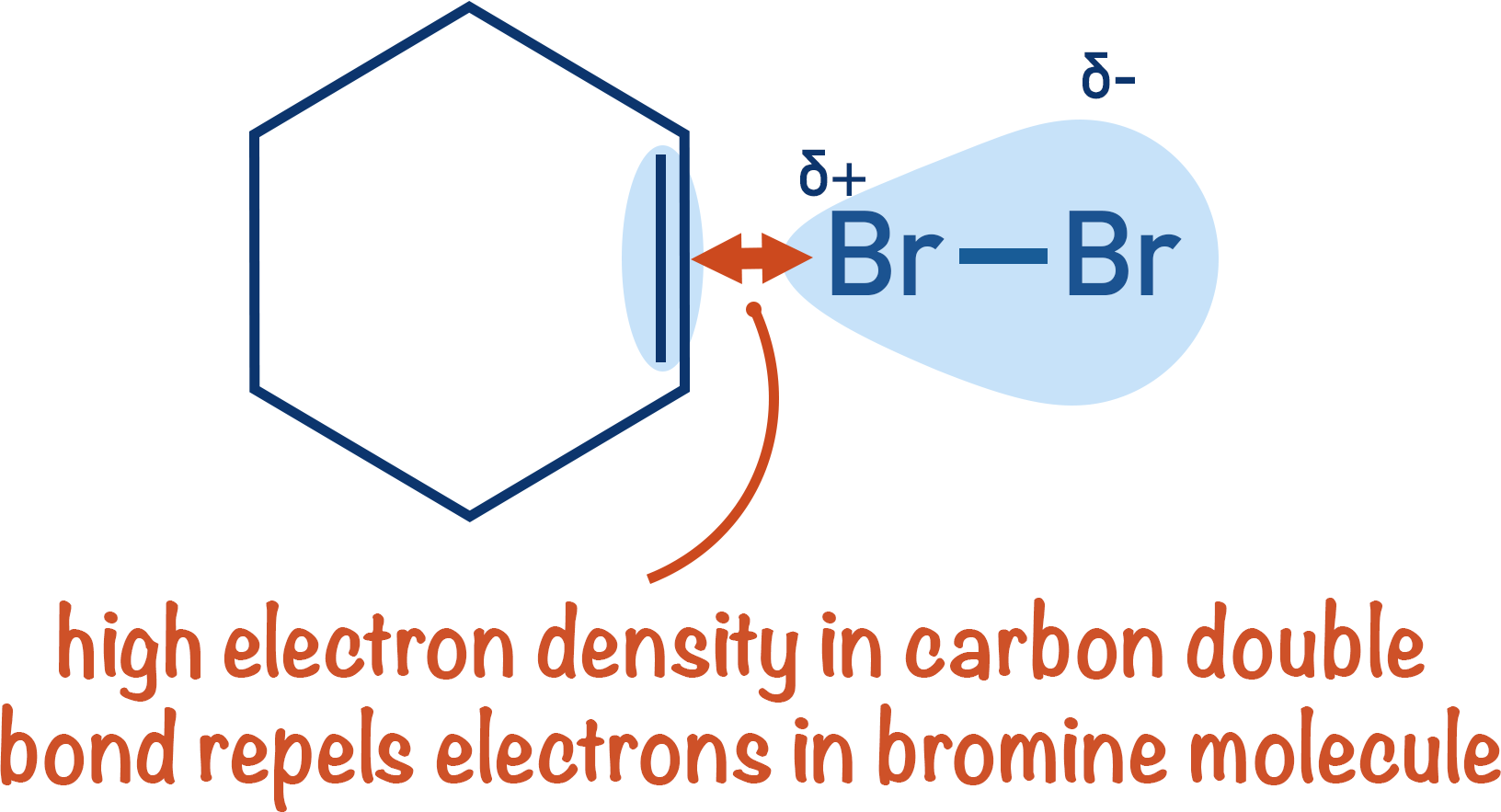
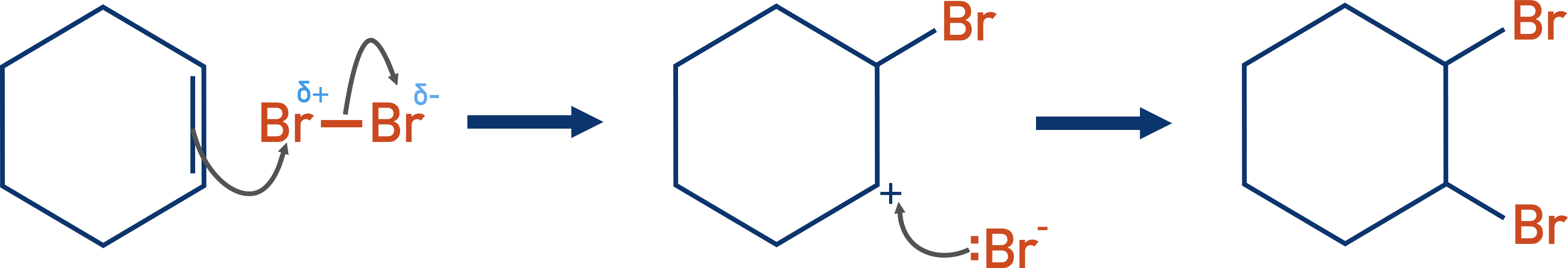
When bromine reacts with an alkene, electrophilic addition occurs. The high electron density in the double carbon bond of the alkene polarises the bromine molecule, enabling an electrophile (Brδ+) to be formed that can react with the double bond.
The electrophile then reacts with the double bond in a standard electrophilic addition reaction.
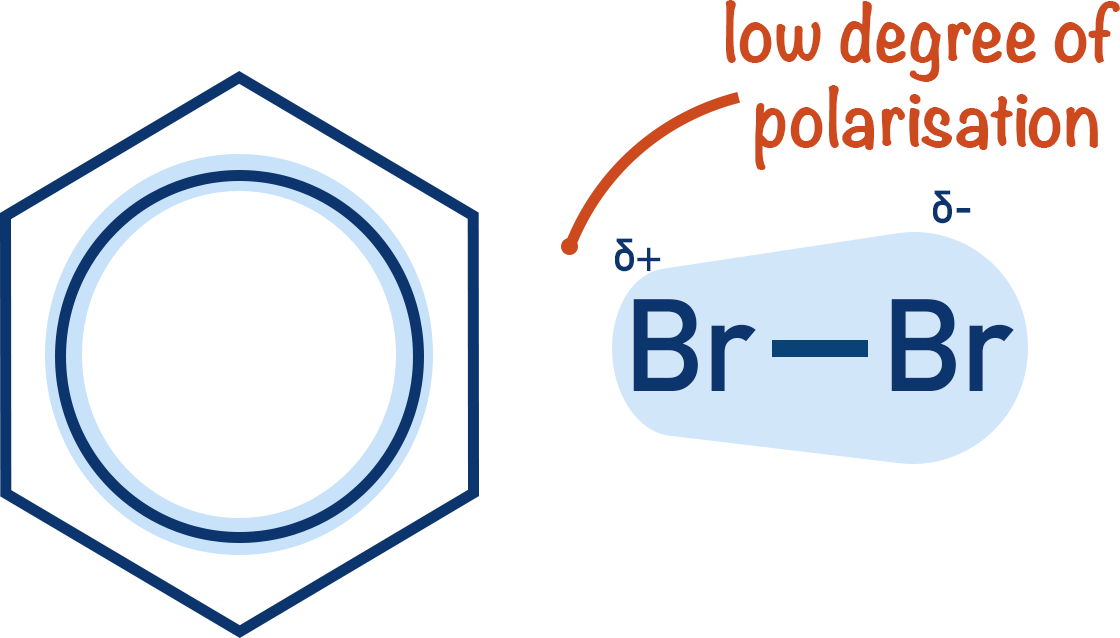
However, when bromine is reacted with a benzene ring no reaction occurs without ‘extra help’. This is because benzene is unable to polarise the bromine molecule enough to produce the Brδ+ electrophile.
As a result, a halogen carrier is needed. A halogen carrier (usually AlBr3) polarises the bond in the bromine molecule sufficiently to allow an electrophilic substitution reaction to occur.
Why is a halogen carrier needed and how is this different to the halogenation of an alkene?
In an alkene, the electrons in the carbon-carbon double bond (pi-bond) are close together (localised) between the two bonding atoms. This means there is a high electron density in this region. This density can polarise the halogen molecule enough to create a halogen electrophile.
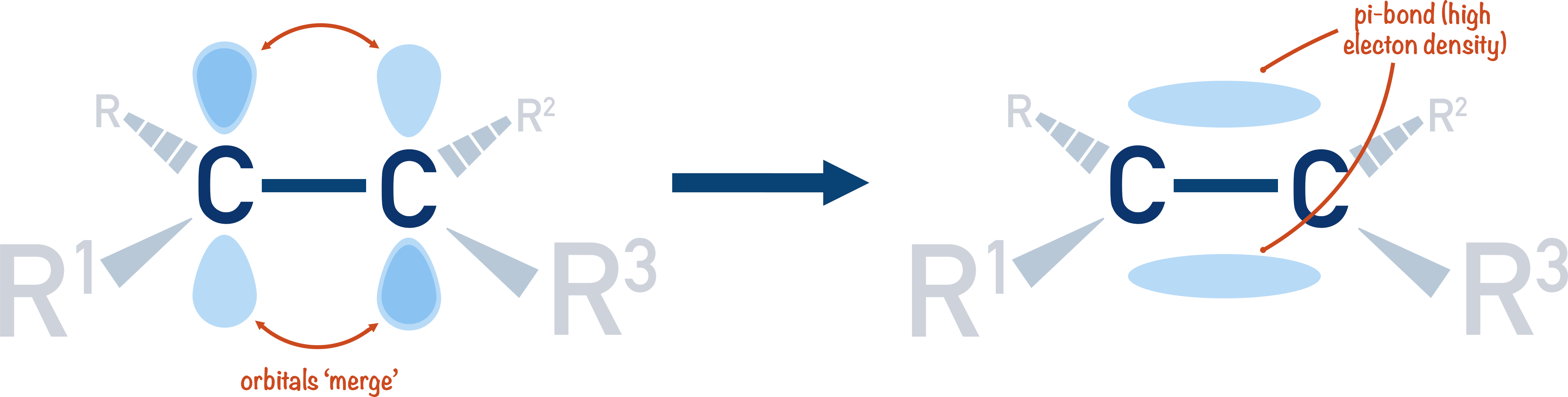
In benzene, however, the electrons that make up the pi-system are not as close together (delocalised).
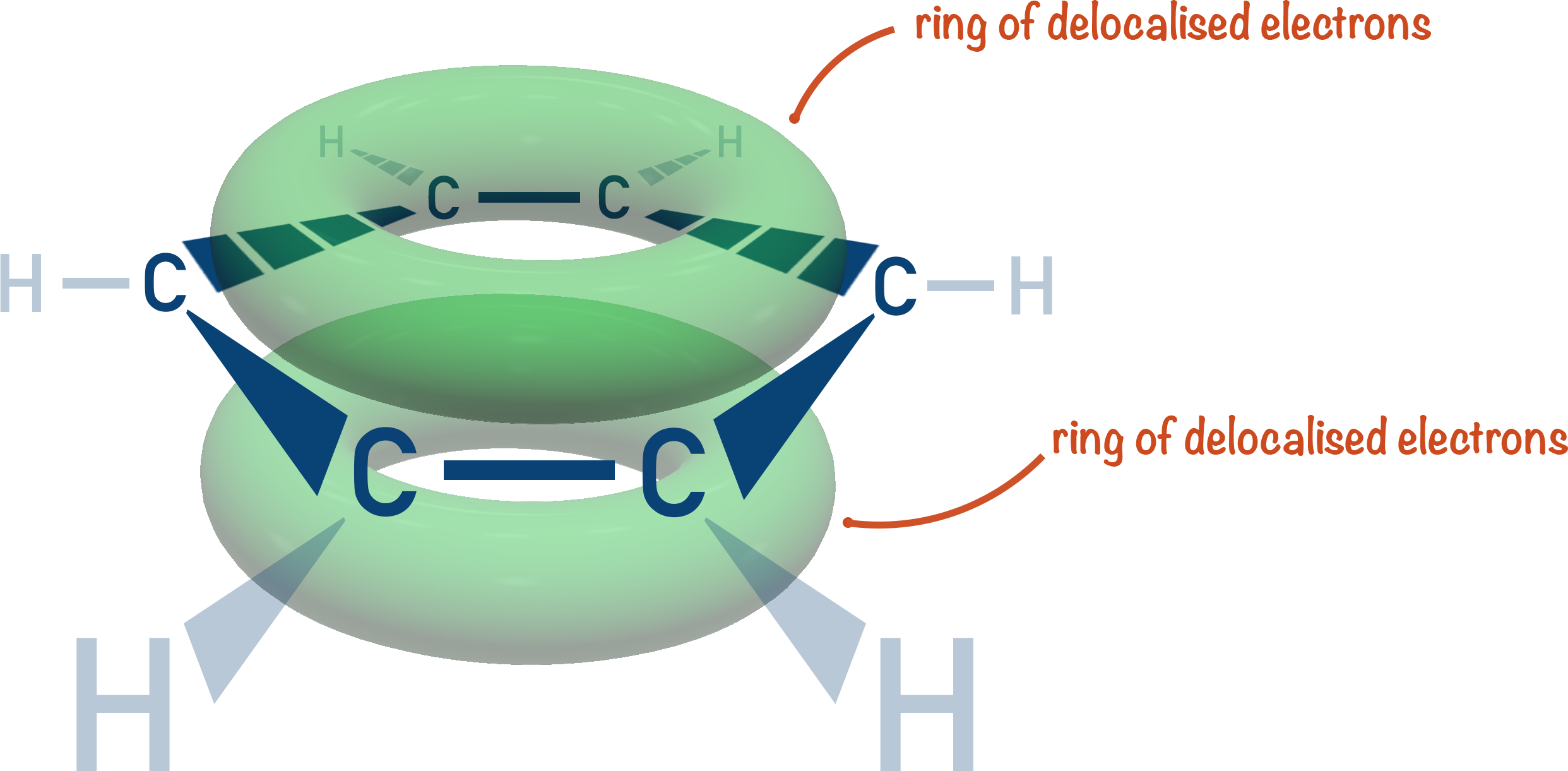
Although there are more pi-bonded electrons in benzene compared to an alkene, they are more spread out. This means when a halogen molecule comes near benzene it’s not polarised as much as if it comes near an alkene. Polarisation is required to create the electrophile.
How does a halogen carrier work?
A halogen carrier is usually made by the reaction of iron or aluminium with the same halogen that is to be added to the benzene ring.
The reaction produces a metal trihalide (iron tribromide (FeBr3) or aluminium trichloride (AlCl3)).
When these species come near a halogen molecule, the positive charge of the metal ion pulls electrons from the halogen molecule towards itself. This causes a large polarisation of the bond within the halogen molecule.
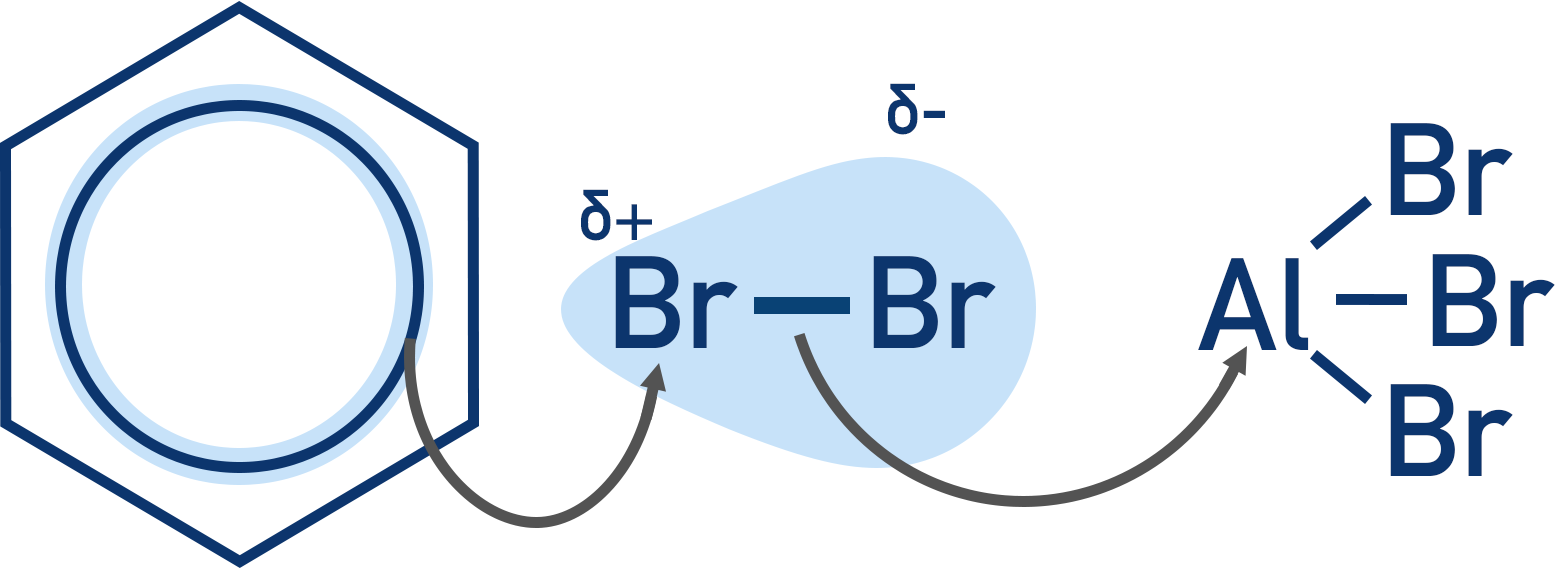
The halogen molecule is now polarised, and the benzene ring can provide just enough extra polarisation to force the molecule to break and form an electrophile that can react with the benzene (electrophilic substitution reaction).
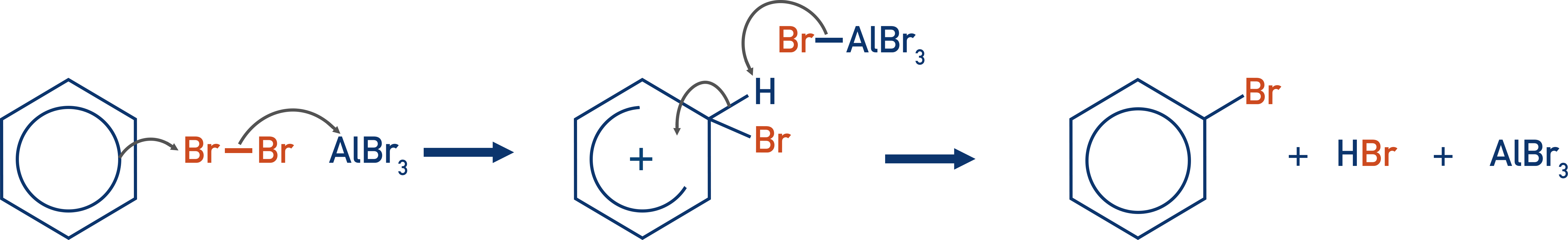
We’ve launched our new site! 🎉
Course-specific notes with built-in search!
AP • A-Level (AQA • CIE • Edexcel • OCR) • IB • NCERT 11 + 12
over 750+ new pages and 3,500 images.
Visit the new homepage
