VSEPR and Hybridization
Quick Notes
- VSEPR theory predicts molecular geometry by minimizing electron pair repulsion.
- Electron pairs (bonding and lone pairs) arrange themselves as far apart as possible.
- Common geometries: linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, trigonal bipyramidal, seesaw, T-shaped, octahedral, square pyramidal, square planar.
- Bond angles depend on geometry and lone pair repulsion.
- Bond order affects bond length and bond energy: higher bond order = shorter, stronger bond.
- Hybridization is the process by which atomic orbitals combine to form new hybrid orbitals for bonding and is linked to geometry:
- sp hybridized → 180° bond angle
- sp² hybridized→ 120° bond angles
- sp³ hybridized→ 109.5° bond angles
- Sigma (σ) bonds = single bonds, formed by direct overlap of orbitals - stronger.
- Pi (π) bonds = found in double/triple bonds, formed by sideways overlap of p-shaped orbitals, weaker, restricted rotation.
- Molecular polarity depends on bond polarity and shape.
Full Notes
To predict the shape and bonding properties of molecules, we combine information from Lewis structures, VSEPR theory, bond order, and electronegativity. These models help explain molecular geometry, bonding patterns, and electron behavior in both molecules and ions.
VSEPR Theory (Valence Shell Electron Pair Repulsion)
The shape of a molecule is determined by repulsions between regions of electron density (electron domains) around the central atom. These regions include:
- Bonding pairs of electrons (in covalent bonds)
- Lone pairs of non-bonded electrons
Key principles:
- Electron pairs repel each other and arrange themselves as far apart as possible in 3D space.
- Lone pairs repel more strongly than bonding pairs because they are closer to the nucleus and occupy more space.
- These repulsions lead to predictable molecular geometries.

Double and triple bonds count as one region of electron density in VSEPR, but they exert slightly stronger repulsion than single bonds, which may reduce adjacent bond angles slightly.
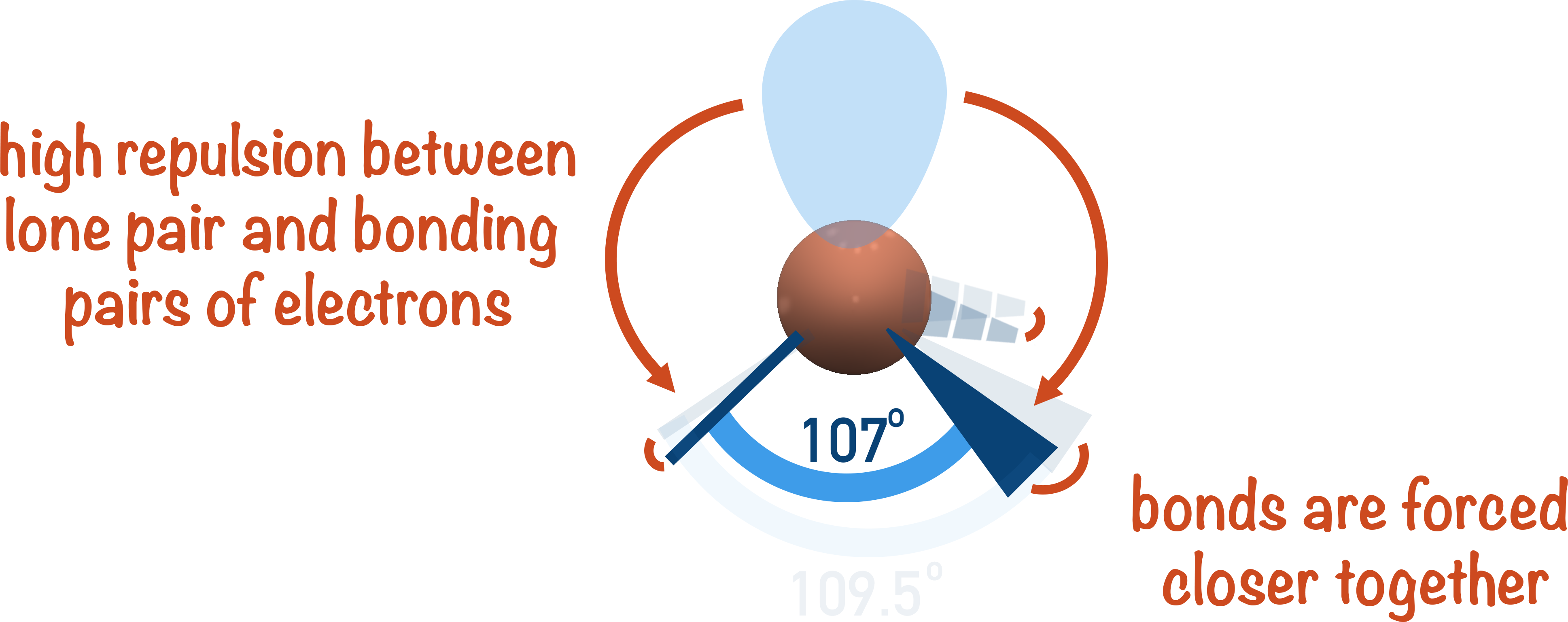
How Lone Pairs Affect Bond Angles
Lone pairs occupy more space than bonding pairs, causing greater repulsion. This pushes bonding pairs closer together and reduces bond angles – the more lone pairs present, the greater the compression.
Common Molecular Shapes and Bond Angles
Linear (180° Bond Angle)

2 bonding pairs, no lone pairs → Equal repulsion forces keep the bonds in a straight line.
Examples:CO2 (O=C=O), BeCl2.
Trigonal Planar (120° Bond Angle)
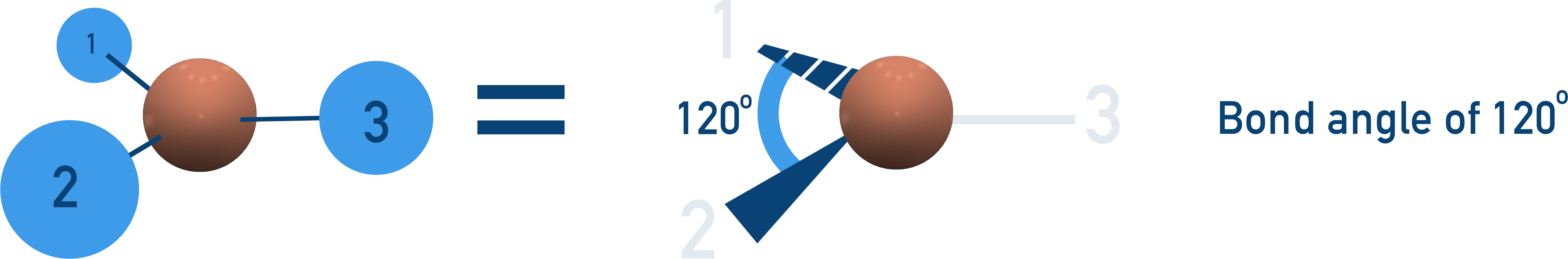
3 bonding pairs, no lone pairs → Electrons spread out evenly in a flat triangle.
Examples:BF3, NO3−.
Tetrahedral (109.5° Bond Angle)
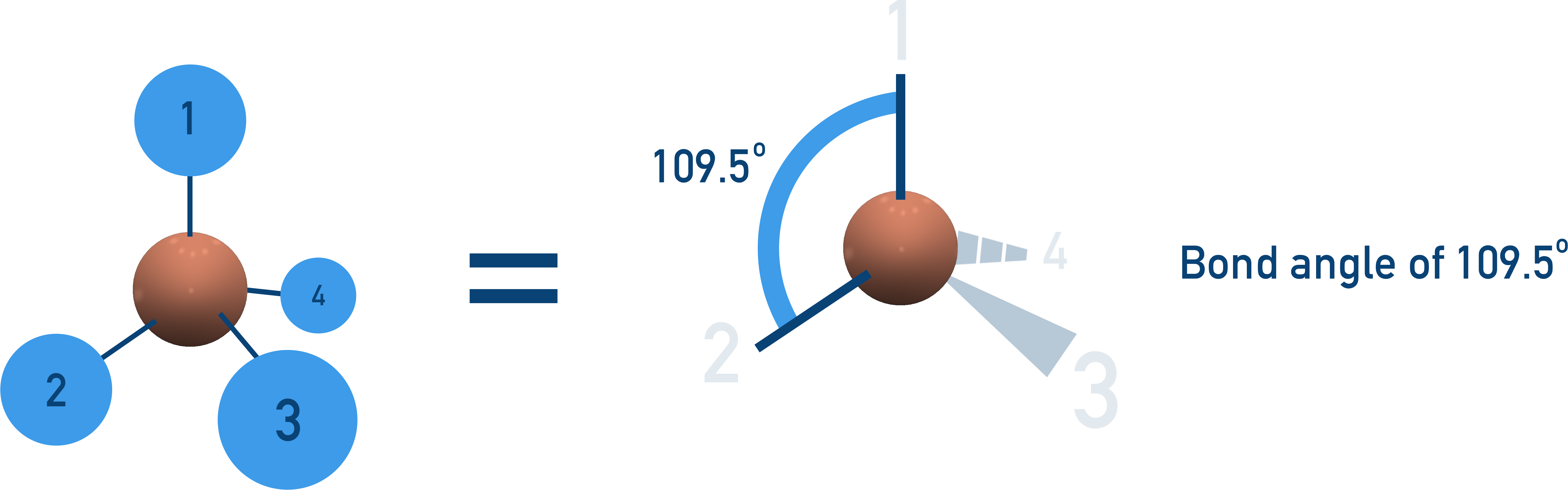
4 bonding pairs, no lone pairs → Electrons arrange in a 3D tetrahedral shape.
Examples:CH4, NH4+.
Trigonal Pyramidal (107° Bond Angle)
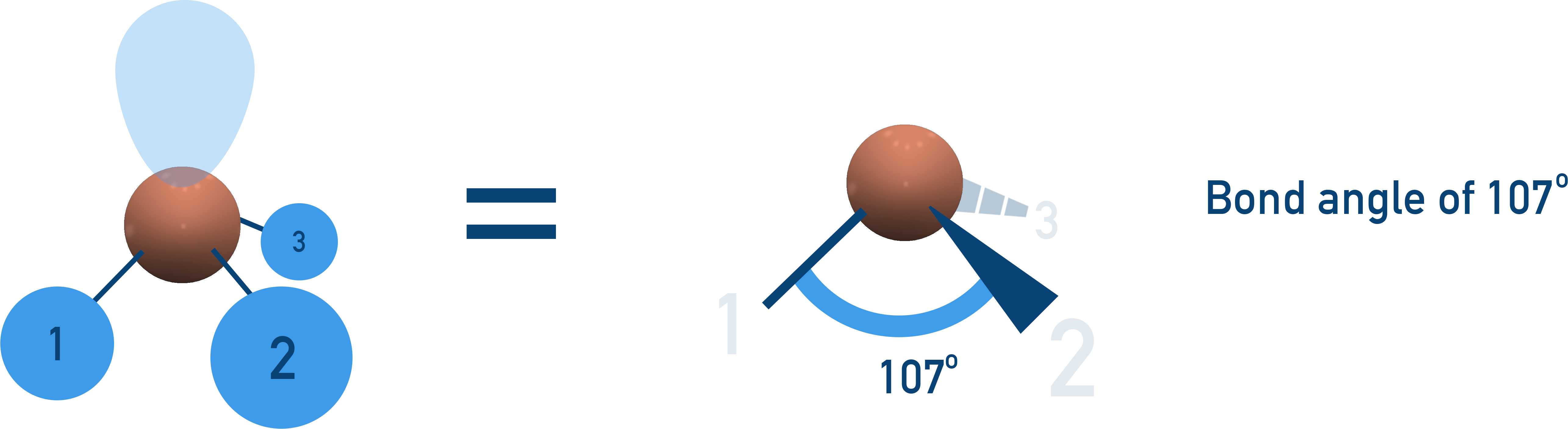
3 bonding pairs, 1 lone pair → Lone pair repulsion reduces bond angle from 109.5° to 107°.
Examples:NH3, PCl3.
Non-linear (104.5° Bond Angle)
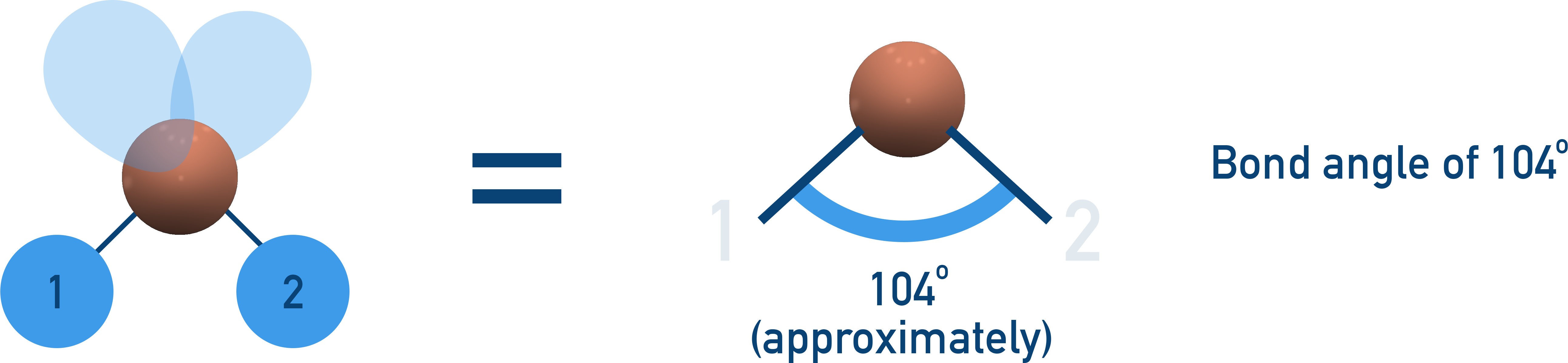
2 bonding pairs, 2 lone pairs → Extra lone pair repulsion reduces bond angle further to 104.5°.
Examples:H2O, OF2.
Trigonal Bipyramidal (90° & 120° Bond Angles)
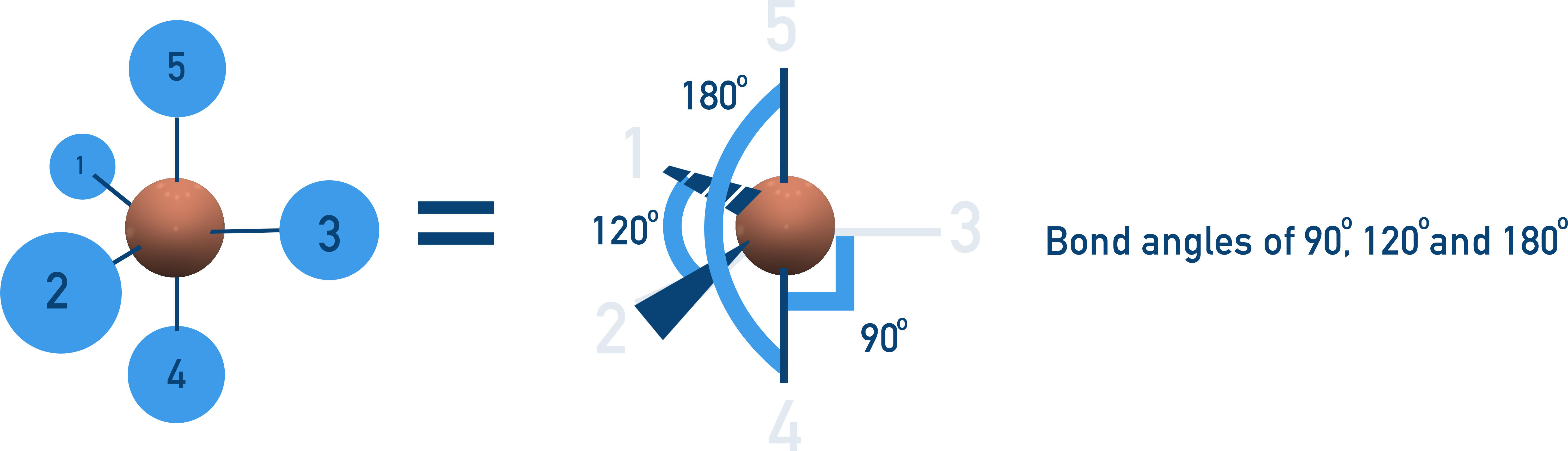
5 bonding pairs, no lone pairs → Atoms arrange in a 3D two-layer shape.
Example:PCl5.
Octahedral (90° Bond Angle)
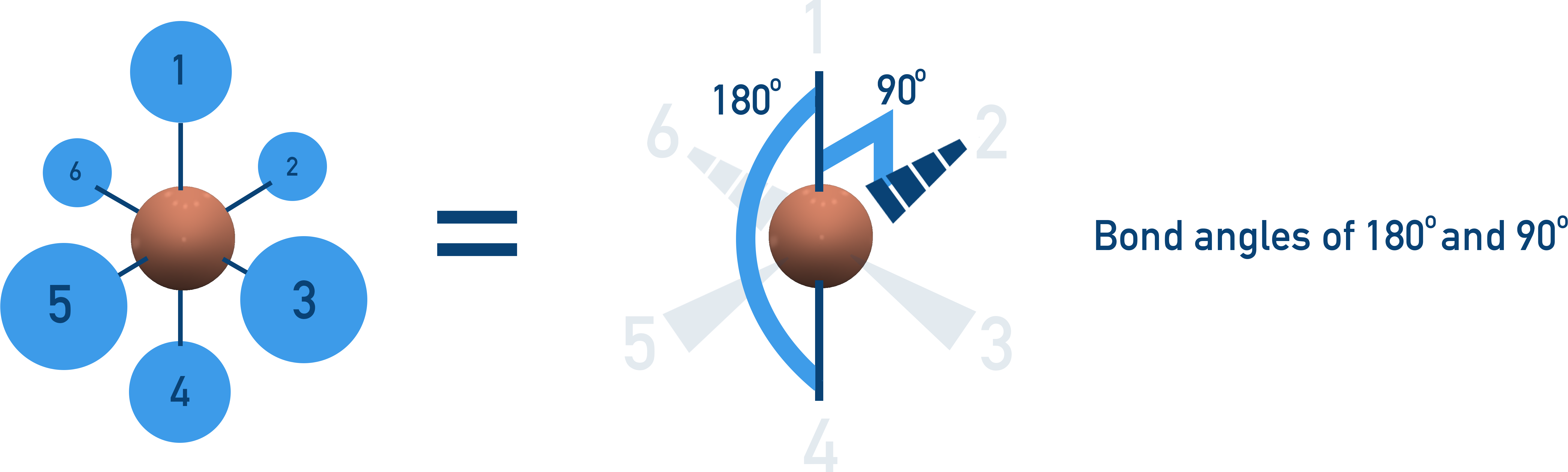
6 bonding pairs, no lone pairs → Electrons spread out in a symmetrical 3D shape.
Example:SF6.
Square Planar (90° Bond Angle)
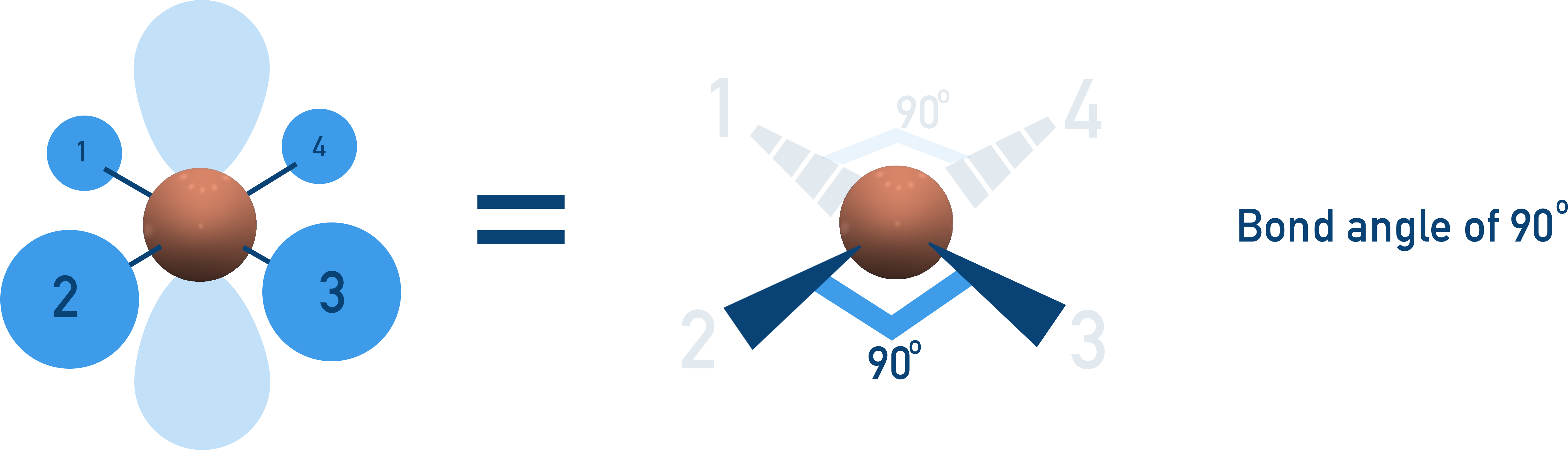
4 bonding pairs, 2 lone pairs → Electrons spread out in a flat, planar arrangement.
Example:[Ni(CN)4]2−.
Application in Ions
The same rules apply for polyatomic ions:
NH4+ → Tetrahedral (109.5°).
NO3− → Trigonal Planar (120°).
OH− → Bent (~104.5°).
Recap - Bond Order, Bond Length, and Bond Strength
Bond order refers to the number of electron pairs shared between two atoms:
- Single bond = 1 shared pair → bond order = 1
- Double bond = 2 shared pairs → bond order = 2
- Triple bond = 3 shared pairs → bond order = 3
As bond order increases:
- Bond length decreases
- Bond strength (bond energy) increases
Why?
More shared electrons lead to greater electrostatic attraction between the negatively charged electrons and both nuclei.
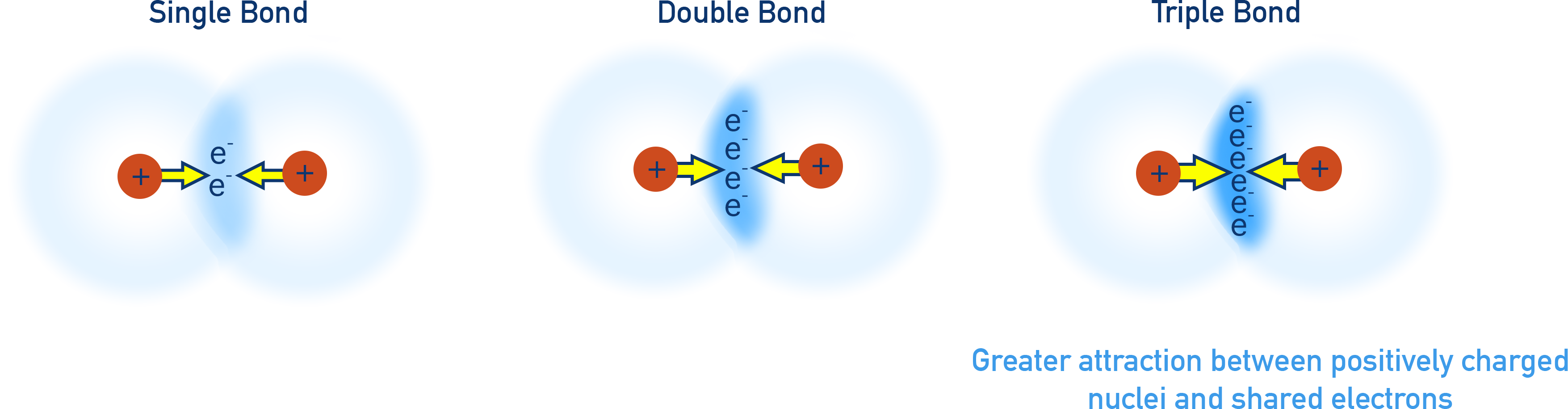
This stronger attraction brings the nuclei closer together (shorter bond) and requires more energy to break (stronger bond).
Molecular Polarity
A molecule can be polar or non-polar, depending on whether it contains polar bonds and the molecules symmetry. Remember a bond can be polar if the two bonding atoms have different electronegativities (see electronegativity).
1. Non-Polar molecules have no permanent dipole
If polar bonds are arranged symmetrically, dipoles cancel out → Non-polar molecule.
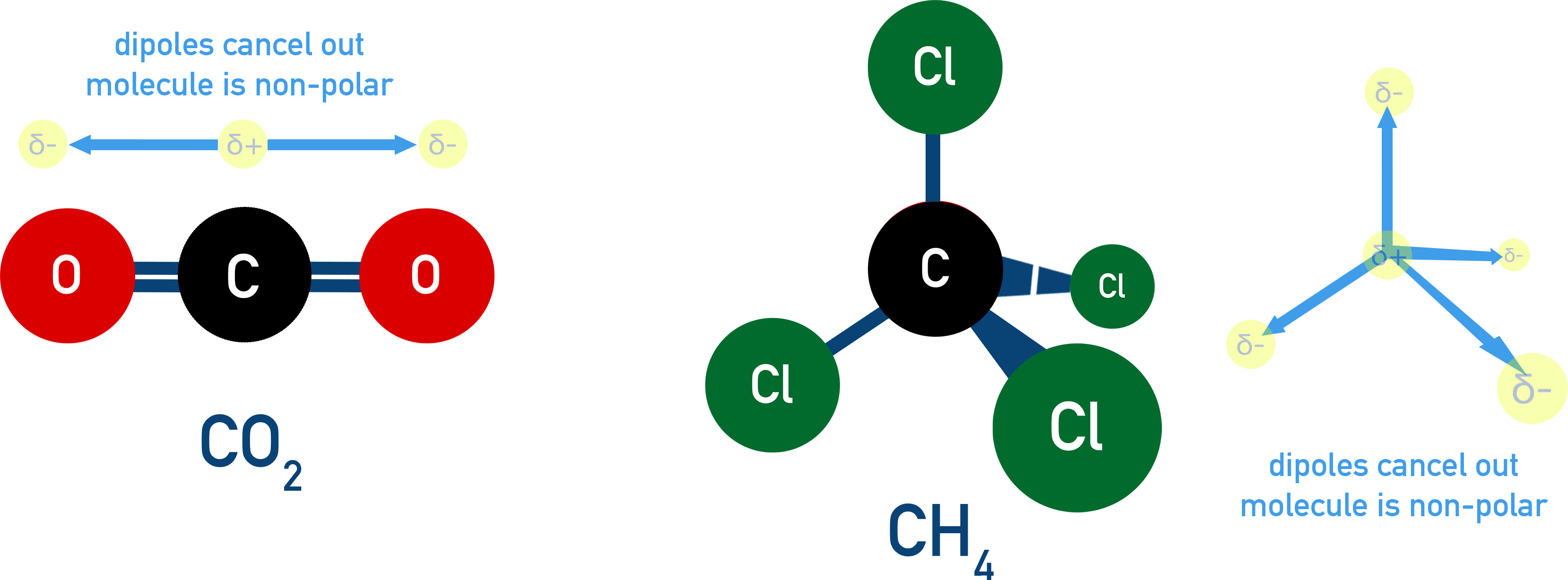
Example CO2 (O=C=O)
Each C=O bond is polar, but the molecule is linear, so dipoles cancel.
No overall dipole = Non-polar molecule.
Example CCl4 (Tetrachloromethane)
Each C–Cl bond is polar, but tetrahedral shape means dipoles cancel.
CCl4 is non-polar despite having polar bonds.
2. Polar molecules have a permanent dipole
If dipoles do not cancel due to asymmetry, the molecule is polar.
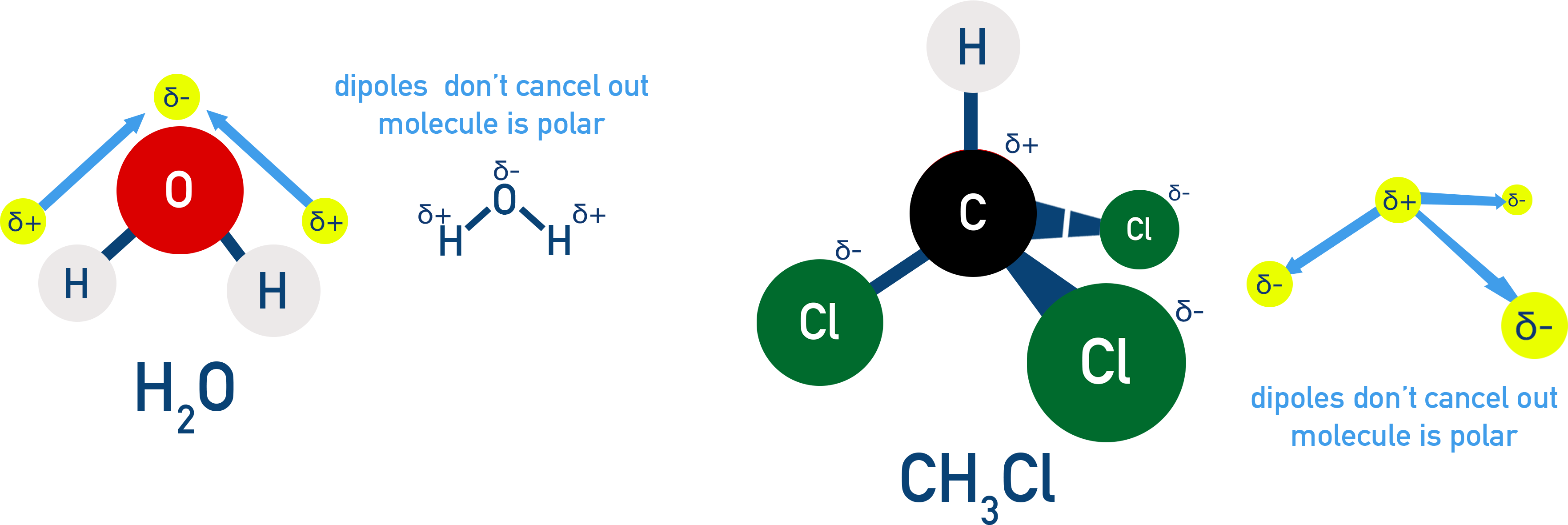
Example H2O
O–H bonds are polar and form a bent shape (104.5°). Dipoles do not cancel → Water is polar.
Example CHCl3 (Chloroform)
The C–H and C–Cl bonds have different polarities. Dipoles do not cancel → CHCl3 is polar.
Hybridization of Atomic Orbitals
Hybridization is the process by which atomic orbitals (s, p) combine to form new hybrid orbitals for bonding. These hybrid orbitals have equal energy and are oriented in specific geometries to minimise electron repulsion. It explains shapes predicted by VSEPR theory and observed experimentally.
Determining Type of Hybridization
To determine hybridization:
- Count the number of electron domains (regions of electron density) around the atom.
- This includes single, double, and triple bonds, as well as lone pairs.
- Match this number to the hybridization.
- 4 regions = sp3
- 3 regions = sp2
- 2 regions = sp
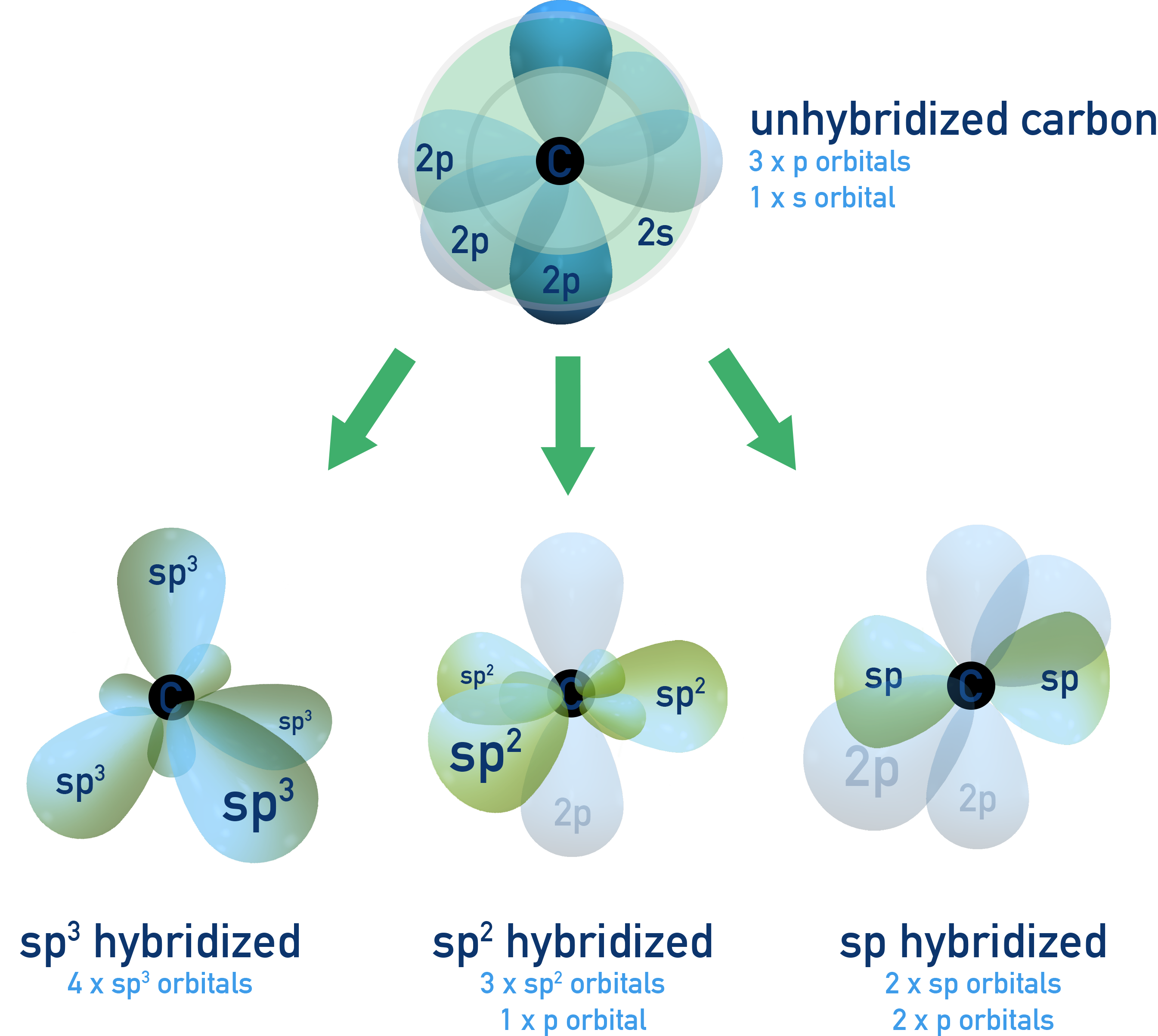
Hybridisation can be used to explain why atoms bond in the way they do (for example, why carbon atoms form four covalent bonds).
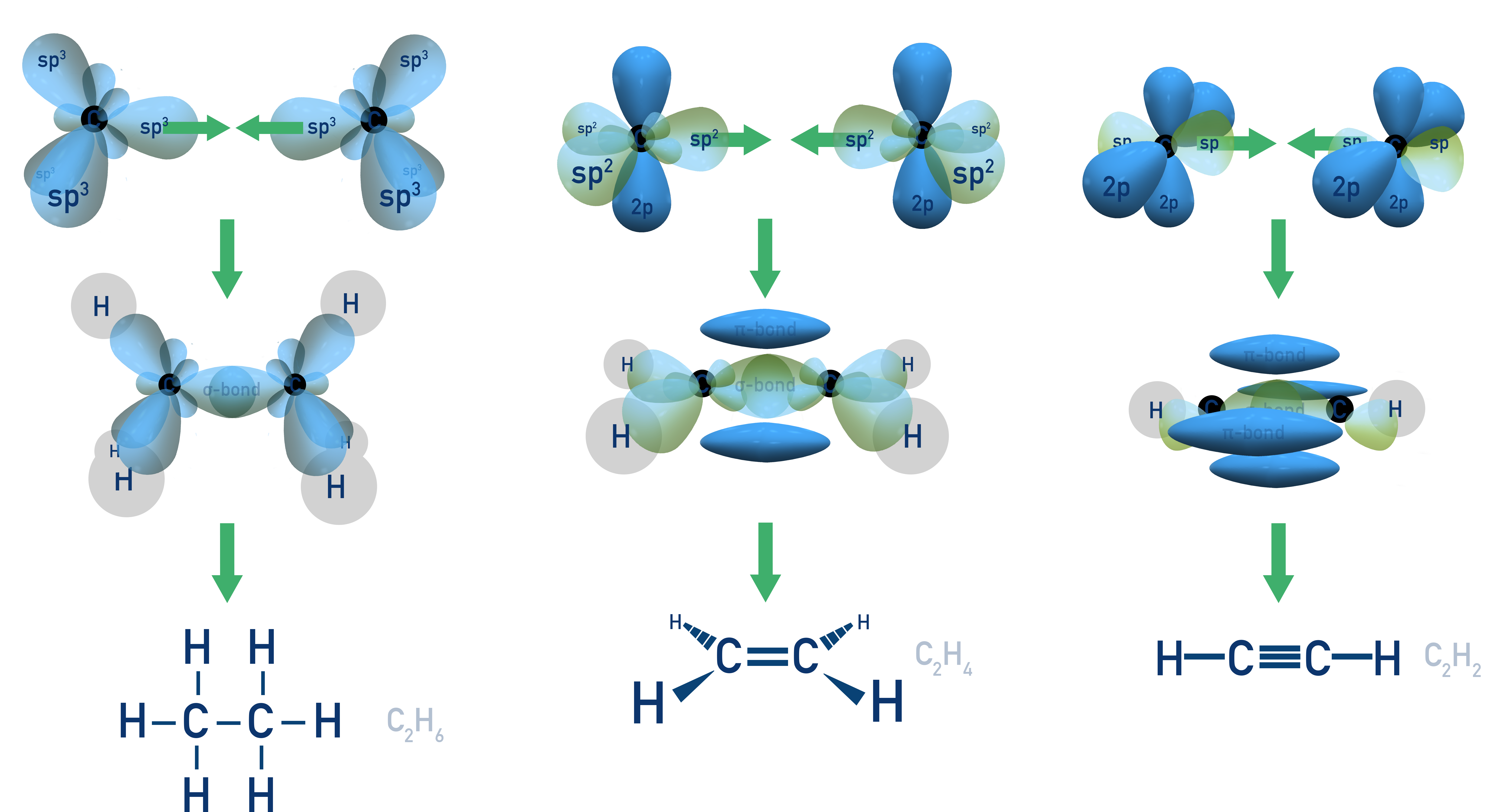
Note: You are not required to understand d-orbital hybridization for the AP Exam.
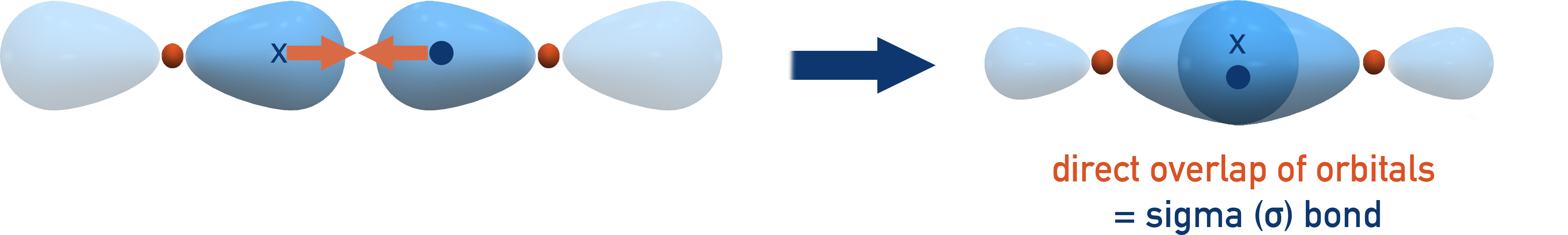
Sigma (σ) and Pi (π) Bonds
σ (sigma) bonds:
Covalent bonds formed by direct (end-to-end) overlap of orbitals along the bond axis. Present in all covalent bonds.
π (pi) bond:
Covalent bonds formed by sideways overlap of adjacent p orbitals above and below the bonding axis. Only found in double and triple bonds.
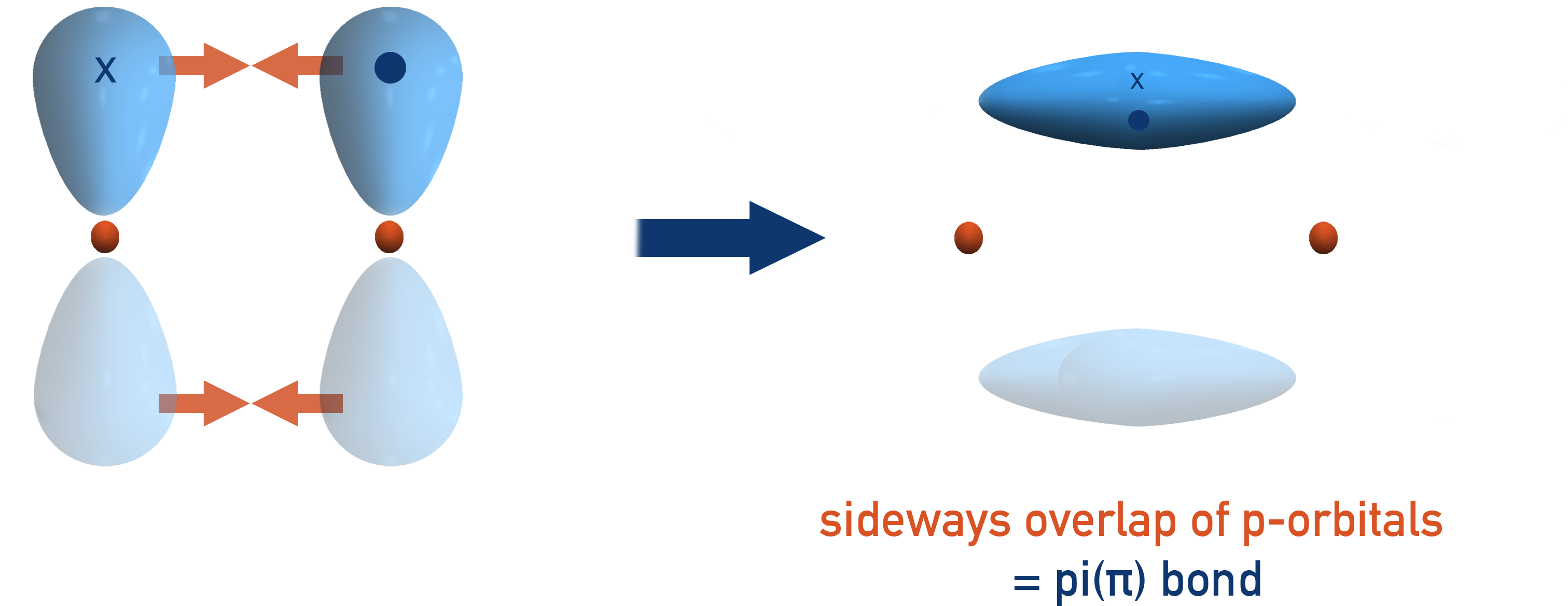
Note the orbital overlap is stronger in sigma than pi bonds, which is reflected in sigma bonds having greater bond energy than pi bonds. Pi bonds restrict rotation around the bond axis. This explains the existence of geometric (cis-trans) isomerism in alkenes.
Determining Number of Sigma and Pi Bonds
- Each single bond is 1 σ
- Each double bond = 1 σ + 1 π
- Each triple bond = 1 σ + 2 π
Examples
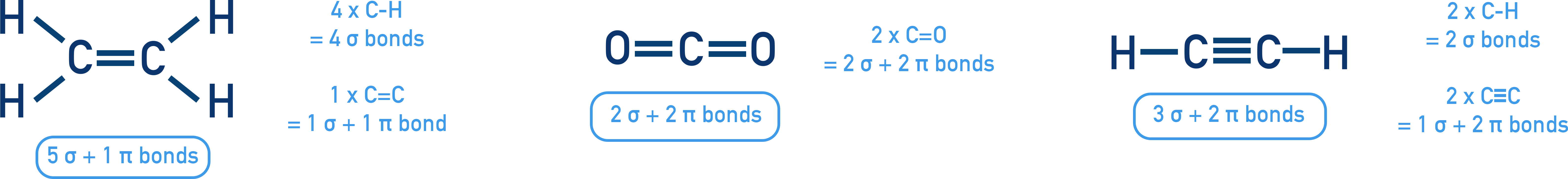 C2H4 (ethene)
C2H4 (ethene)
Structure: H–C=C–H
5 σ bonds (1 C–C and 4 C–H)
1 π bonds (from C=C double bond)
CO2
O=C=O
2 double bonds → 2 σ + 2 π
C2H2 (ethyne)
Structure: H–C≡C–H
3 σ bonds (1 C–C and 2 C–H)
2 π bonds (from C≡C triple bond)

Be careful to distinguish electron geometry (based on all regions) from molecular shape (only bonded atoms). Always count total electron regions and match the hybridization accordingly.
Summary
- VSEPR theory and hybridization help us predict the structure and bonding in molecules. Molecular shape depends on the number of electron regions around the central atom.
- Hybridization gives insight into the types of orbitals involved in bonding.
- Bond polarity, bond order, and molecular geometry together determine a molecule’s polarity and reactivity.
- The presence of pi bonds also explains properties like restricted rotation and multiple bond strength.
- Key points to remember:
- VSEPR: electron pairs repel → shape predicts geometry
- Lone pairs affect bond angles and shape
- Hybridization: sp (2 regions), sp² (3), sp³ (4)
- Sigma (σ) = all single bonds and the first bond in multiple bonds
- Pi (π) = the second and third bonds in double/triple bonds
