Molecular Structure of Acids and Bases
Quick Notes
- Acid/base strength is directly related to the stability of the conjugate base or acid.
- Strong acids have very stable conjugate bases.
- Electronegativity, resonance, inductive effects, and bond strength affect acid strength.
- Carboxylic acids are common weak acids.
- Nitrogen-based compounds (like ammonia) are typical weak bases.
Full Notes
What Determines Acid Strength?
The strength of an acid depends on how readily it donates a proton (H+). This is influenced by two key factors:
- The polarity and strength of the H–X bond (how easily the bond breaks)
- The stability of the conjugate base (A−) once the proton is lost
The more stable the conjugate base, the more the HA equilibrium (HA ⇌ H+ + A-) favors dissociation – and the stronger the acid.
Strong acids like HCl, HBr, HI, HNO3, H2SO4, and HClO4 fully dissociate in water. Their conjugate bases (e.g., Cl−, NO3−) are very stable, so they do not re-accept the proton, keeping the acid molecules dissociated in solution.
There are several key factors that affect conjugate base stability.
1. Electronegativity
A conjugate base is more stable if the negative charge is held by a more electronegative atom.
Example: HCl → H+ + Cl−
Chlorine is highly electronegative, so Cl− holds the negative charge.

2. Resonance Delocalization
Conjugate bases that delocalize the negative charge across multiple atoms are more stable.
Example: Carboxylic acids (R–COOH) form the conjugate base R–COO−. The negative charge on COO− is spread over two oxygen atoms via resonance, making it more stable and enhancing acidity.
Carbonate ion (CO32−)
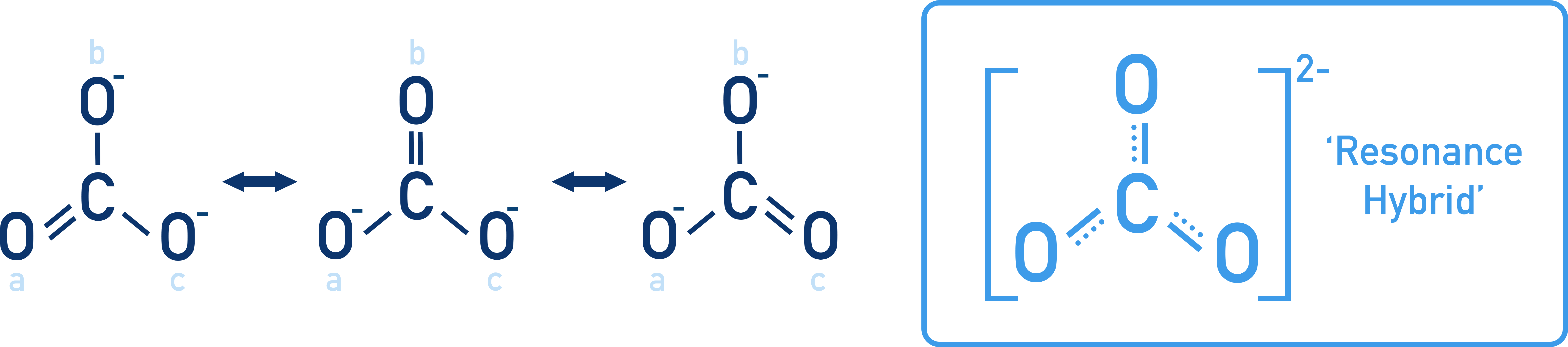
Three resonance structures, each with one C=O and two C–O− bonds
3. Inductive Effects
Electronegative atoms near the acidic proton (the H that gets donated by the acid) pull electron density away, polarizing the bond and making the H+ easier to remove.
For Example: In H2SO4, the presence of several electronegative oxygen atoms draws electron density away from the O–H bond, polarizing and weakening it, making it easier to break.
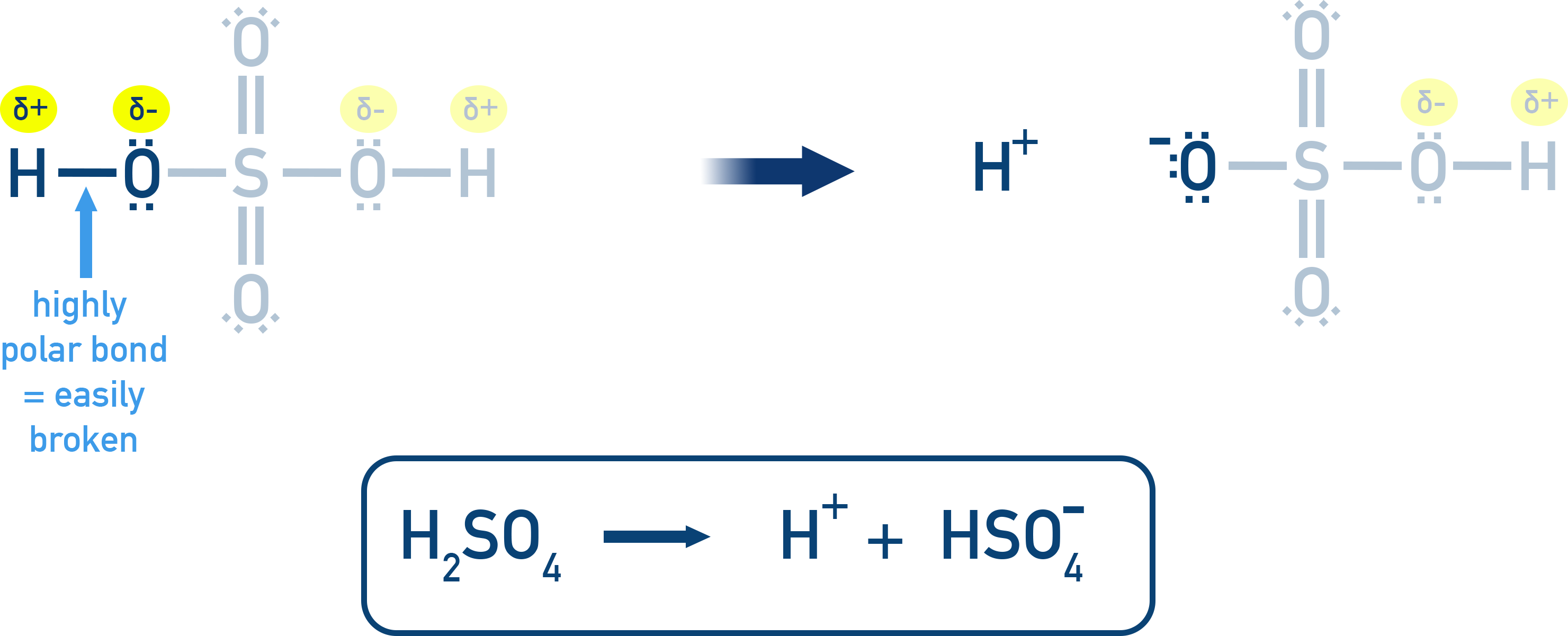
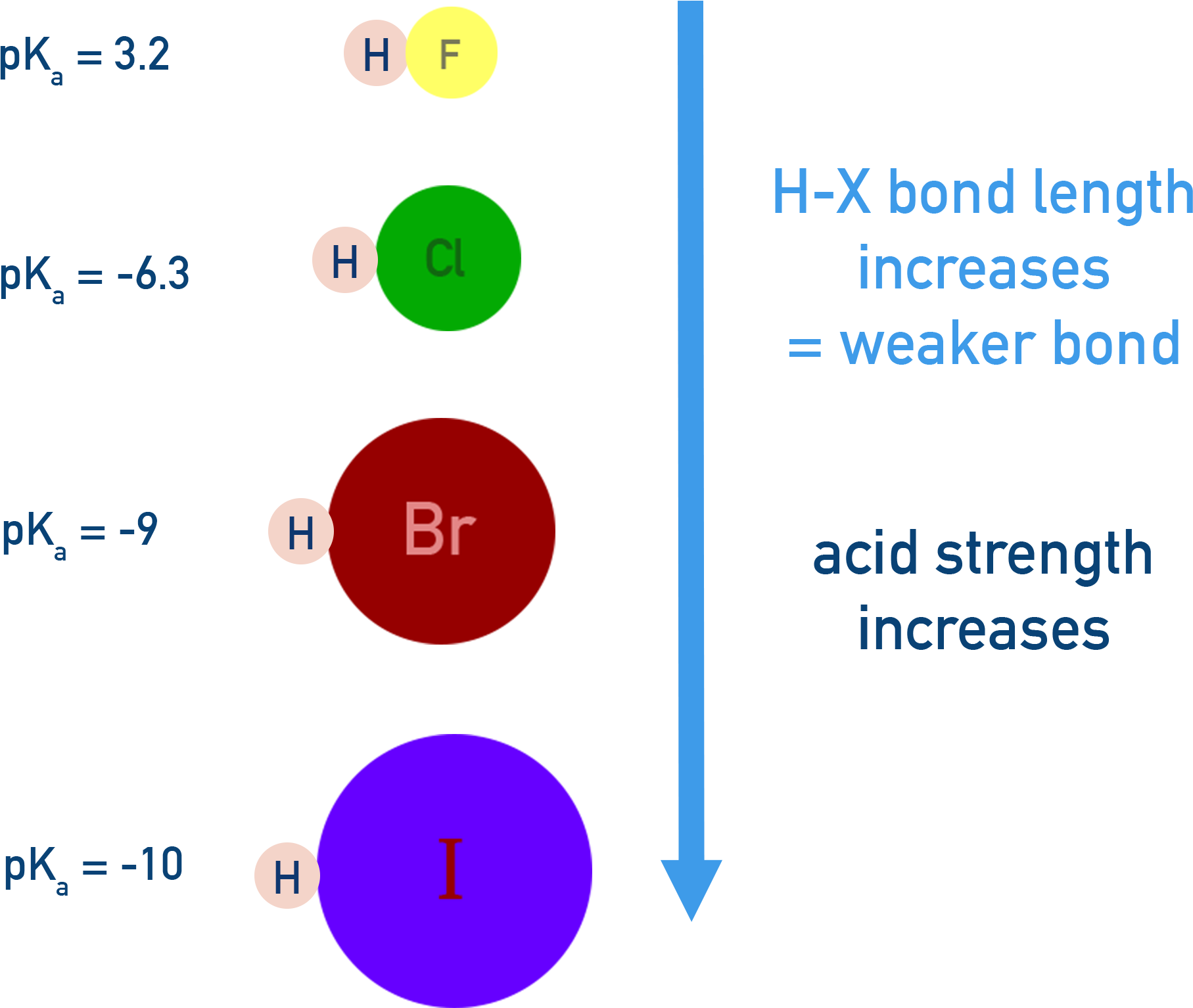
4. Bond Strength (Bond Enthalpy)
Weaker H–X bonds are easier to break, increasing acid strength — even if electronegativity is lower.
For Example:
Among hydrogen halides: H–I < H–Br < H–Cl (in bond strength)
HI is the strongest acid because the H–I bond is weakest and I− is a large, stable ion.
Acid and Base Types and Their Conjugates
Strong Acids Have Very Weak Conjugate Bases
Strong acids dissociate completely, and their conjugate bases are extremely weak—often inert in water.
Examples:
- HCl → Cl−
- HNO3 → NO3−
- H2SO4 → HSO4−
These anions are stabilized by electronegativity or resonance.
Weak Acids Have Weakly Basic Conjugate Bases
Weak acids only partially dissociate. Their conjugate bases may accept H+ ions, often acting as weak bases in equilibrium.
Example:
CH3COOH ⇌ H+ + CH3COO−
The ethanoate ion is stabilized by resonance.
Strong Bases Have Very Weak Conjugate Acids
Strong bases such as Group I/II hydroxides fully dissociate in water to form OH− ions. Their conjugate acids (e.g. Na+, Ca2+) are neutral and do not react with water.
Examples:
- NaOH → Na+ (no effect on pH)
- Ba(OH)2 → Ba2+ (inert cation)
Weak Bases Have Moderately Strong Conjugate Acids
Weak bases partially accept protons. Their conjugate acids may donate protons back, acting as weak to moderate acids.
These are often nitrogen-containing species with lone pairs.
Examples:
- NH3 ⇌ NH4+
- CH3NH2 ⇌ CH3NH3+
- Carboxylate ions like CH3COO− ⇌ CH3COOH
Electronegativity and Acid Strength
Electronegativity helps stabilize the conjugate base, increasing acid strength.
Atoms like F, Cl, and O hold negative charge more effectively. A more stable conjugate base means the acid donates protons more easily. This principle is seen in oxoacids (acids that contain oxygen), where more electronegative atoms or more oxygen atoms increase acidity.
Summary
- Stronger acids have more stable conjugate bases.
- Conjugate base stability is enhanced by:
- Electronegativity (pulls negative charge)
- Resonance (delocalizes charge)
- Inductive effects (spread out charge)
- Weak H–X bonds (easier to break)
- Common strong acids include HCl, HNO3, H2SO4, HClO4
- Carboxylic acids are common weak acids
- Common strong bases include NaOH, KOH, Ba(OH)2
- Common weak bases include NH3, amines, carboxylate anions
