Heat Transfer and Thermal Equilibrium
Quick Notes
- Heat transfer is the movement of thermal energy from one substance to another due to a temperature difference.
- Particles in a warmer substance move faster (higher average kinetic energy).
- When two substances are in contact, collisions between particles allow energy to flow.
- Heat flows from hot to cold until thermal equilibrium is reached.
- At equilibrium, both substances have the same temperature (equal average kinetic energy).
Full Notes
Molecular Motion and Temperature
Temperature is a measure of the average kinetic energy of the particles in a substance.
- In a warmer object, particles move faster.
- In a cooler object, particles move more slowly.
When two objects at different temperatures come into contact, energy is transferred through collisions between particles.
Heat Transfer and Collisions
When a fast-moving particle (from a warmer object) collides with a slower particle (from a cooler object), some energy is transferred.
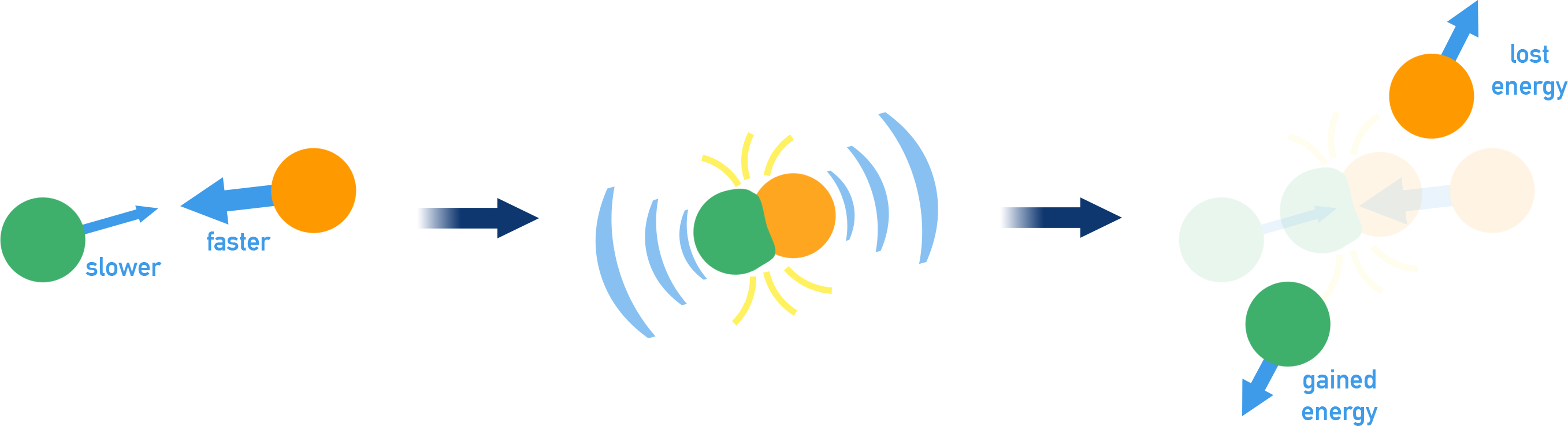
The warmer particle slows down, and the cooler particle speeds up. This process continues with more collisions.
The combined total energy of all particles is conserved but redistributed between them. This flow of energy is called heat transfer (also known as thermal energy transfer or energy exchange).

Heat transfer always occurs spontaneously from higher to lower temperature. Energy does not flow from cold to hot unless work is done (e.g., in a refrigerator).
Thermal Equilibrium
As energy is exchanged between two objects or systems, the temperature difference decreases.
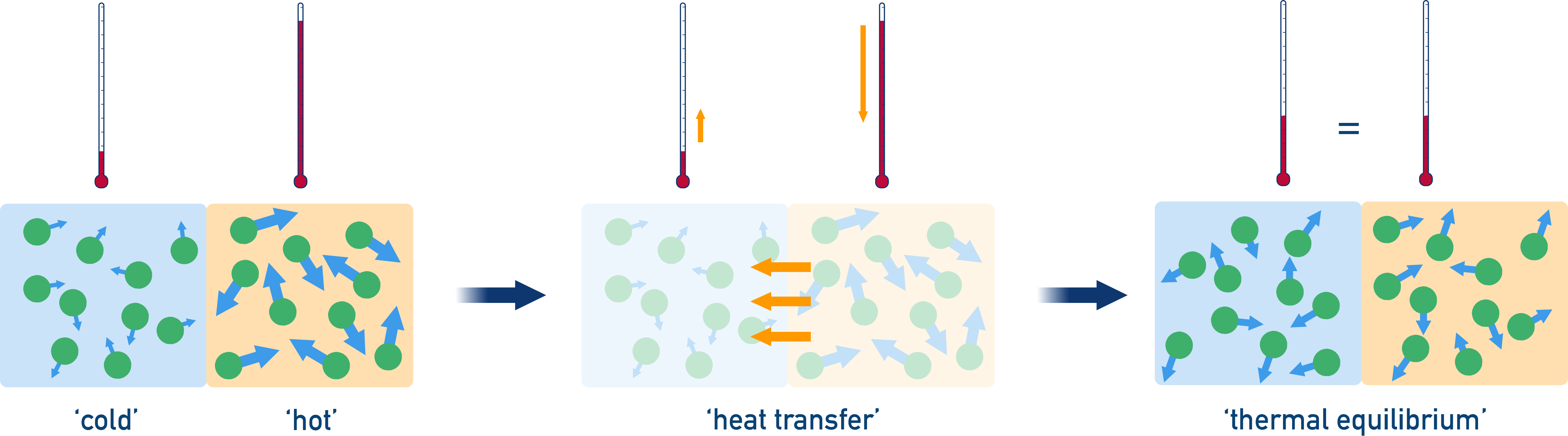
Thermal equilibrium is reached when both substances have the same average kinetic energy, and hence the same temperature.
At this point, there is no net transfer of energy between the systems.
Example:If hot metal is placed in cold water, heat flows from the metal to the water until both reach the same temperature.
Summary
Heat transfer is the result of particle collisions between substances at different temperatures. Particles in the warmer object have higher kinetic energy and transfer energy to cooler particles upon collision.
This exchange continues until both substances reach thermal equilibrium, where their particles have the same average kinetic energy and temperature. Understanding this microscopic mechanism explains how energy moves and helps predict the direction and outcome of thermal interactions.
