Introduction to Enthalpy of Reaction
Quick Notes
- Reactions release or absorb heat energy depending on bond breaking and bond forming.
- Enthalpy change (ΔH) tells us the amount of heat exchanged at constant pressure per mole of reaction.
- Key equation:
q = nΔHr - q = heat (J or kJ)
- n = moles of limiting reactant
- ΔHr = enthalpy change per mole of reaction (J/mol or kJ/mol)
-
where
Full Notes
What Is Enthalpy Change?
The enthalpy change (ΔH) of a reaction describes the amount of heat released or absorbed during a chemical reaction at constant pressure and has units of kJ mol-1.
Exothermic reactions: ΔH is negative (heat is released).
Endothermic reactions: ΔH is positive (heat is absorbed).
In a chemical reaction, bonds are broken in reactants and new bonds are formed in products. These changes affect the chemical potential energy of the system (see Topic 6.7) and the energy difference results in a change in the kinetic energy of the particles, which causes a temperature change.
Calculating Heat from Enthalpy
Once you’ve calculated the heat energy change, q (see Topic 6.4), you can determine the enthalpy change per mole using:
ΔH = −q / n
Where:
- ΔH = enthalpy change per mole (kJ mol⁻¹)
- q = heat energy (convert to kJ if needed)
- n = number of moles of limiting reagent
The negative sign accounts for the fact that q measures the heat gained or lost by the surroundings, whereas ΔH refers to the energy change of the system.
If q is positive (surroundings gain heat), the system has released energy → ΔH is negative (exothermic).
If q is negative (surroundings lose heat), the system has absorbed energy → ΔH is positive (endothermic).
You can also rearrange the formula to calculate q if ΔH and n are known:
q = n × ΔH
This form is useful when ΔH is provided in a reaction equation and you want to find the total heat energy change.

Always match n to the number of moles of the limiting reactant as shown in the balanced chemical equation.
Ensure all units are consistent — particularly converting q to kJ if ΔH is expressed in kJ mol⁻¹.
Understanding the Energy Flow
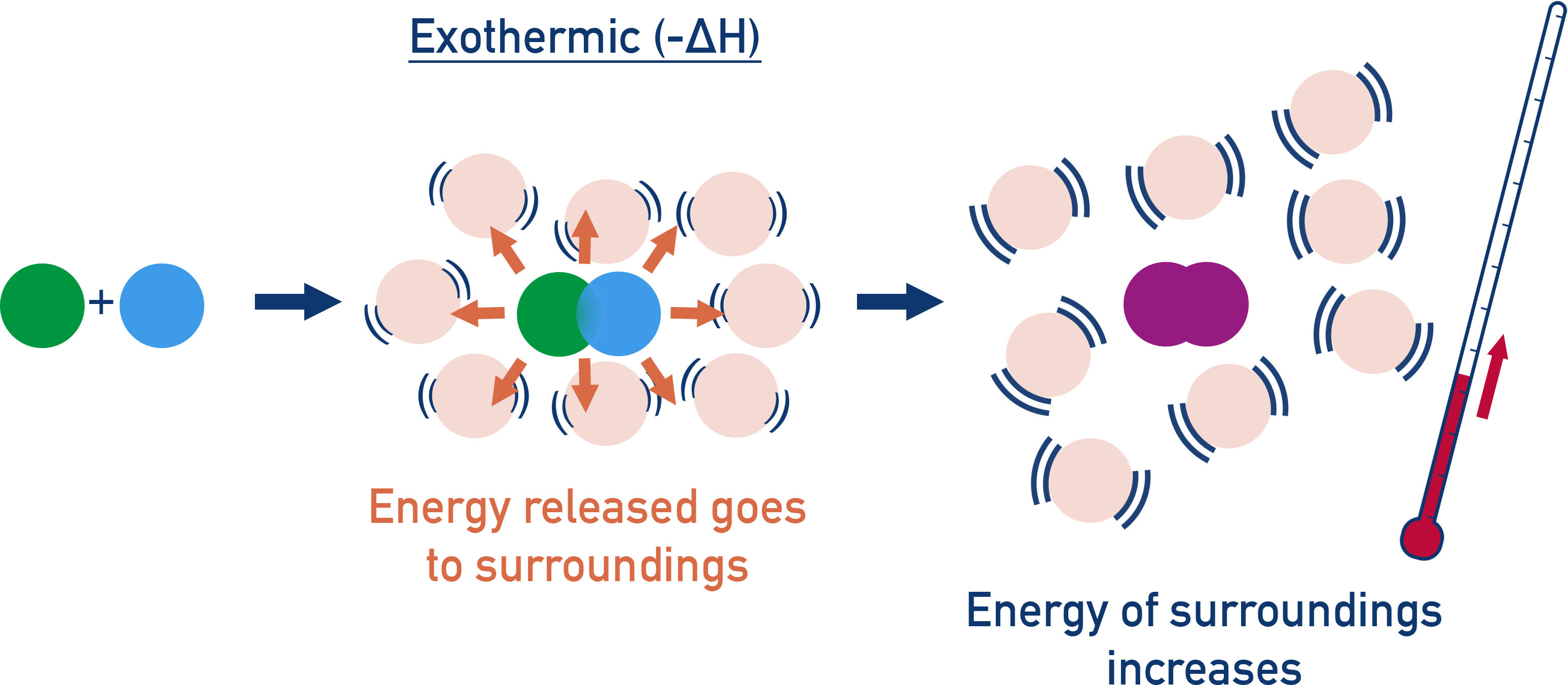
In an exothermic reaction, the system loses energy. Heat flows to the surroundings → temperature of surroundings goes up.
In an endothermic reaction, the system gains energy. Heat flows from the surroundings → temperature of surroundings goes down.
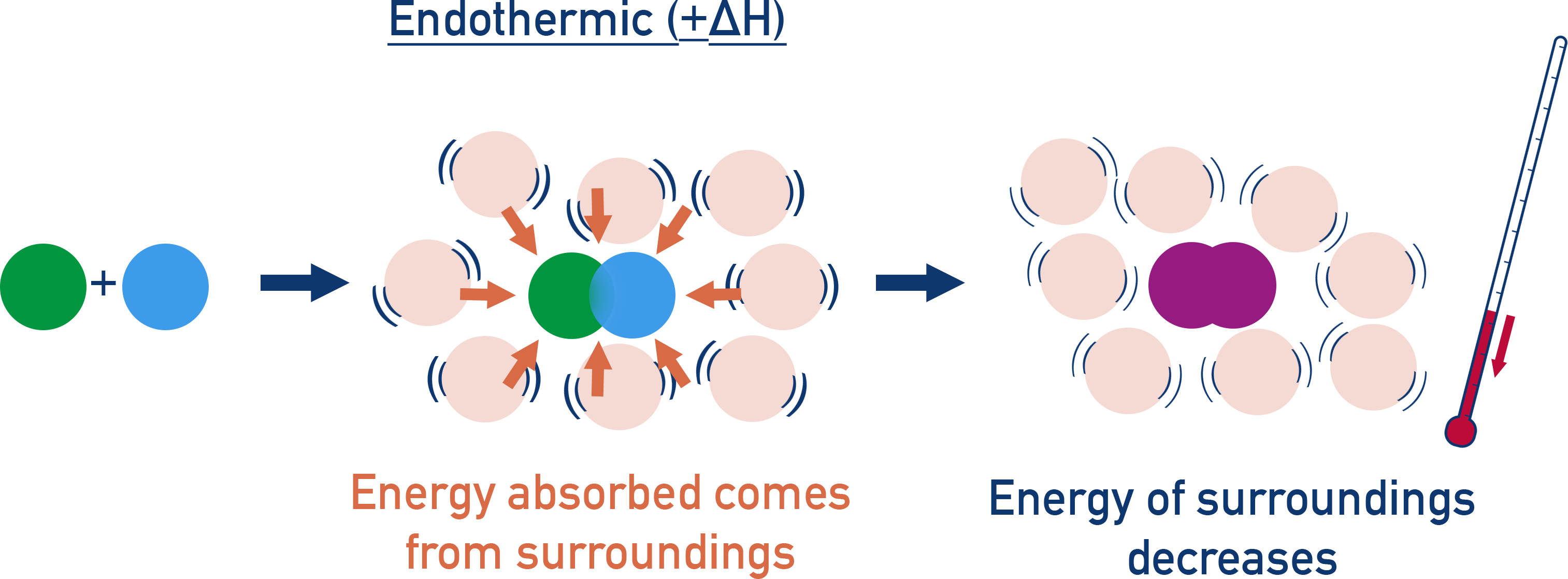
These thermal energy changes are observed as a temperature change in calorimetry experiments, assuming the reaction happens at constant pressure.
Examplewhen burning methane:
CH₄ + 2O₂ → CO₂ + 2H₂O ΔH = −890 kJ/mol
This means that 890 kJ of energy is released when 1 mol of methane reacts.
If you only combust 0.5 mol of CH₄:
q = (0.5 mol)(−890 kJ/mol) = −445 kJ
Summary
The enthalpy change of a chemical reaction represents the heat energy transferred at constant pressure. Use q = nΔHr to calculate the heat exchanged. The sign of ΔH tells you whether the system absorbs or releases energy — which in turn can be observed through a change in temperature of the surroundings.
