Galvanic (Voltaic) and Electrolytic Cells
Quick Notes
- Electrochemical cells convert between chemical energy and electrical energy.
- Galvanic (Voltaic) cells: spontaneous reactions that generate electricity (ΔG < 0).
- Electrolytic cells: non-spontaneous reactions driven by external electrical energy (ΔG > 0).
- Simple terms – Galvanic (Voltaic) cells use a reaction to produce electricity and electrolytic cells use electricity to make a reaction occur.
- In all cells:
- Oxidation occurs at the anode.
- Reduction occurs at the cathode.
Full Notes
Introduction: What Is an Electrochemical Cell?
Electrochemical cells use redox (reduction–oxidation) reactions to either produce electricity or use electricity to drive chemical changes. They are essential for understanding how chemical energy is converted into electrical energy – and vice versa – through the movement of electrons and ions.
- Voltaic (Galvanic) cells generate electricity from a spontaneous redox reaction.
- Electrolytic cells use electricity to force a non-spontaneous redox reaction to occur.
Both of these are outlined briefly below and then in further detail (if you are interested) at the bottom of this page. I suggest you make sure you are happy with exactly how they work before progressing onto topic 9.9, Cell Potential and Free Energy.
Core Idea: Where Does Redox Happen?
All electrochemical cells contain two solid electrodes placed into a liquid (electrolyte).
- The anode is the electrode where Oxidation always happens.
- The cathode is the electrode where Reduction always happens (remember oxidation = loss of electrons, reduction = gain of electrons).
- The sign (positive/negative) of each electrode depends on the type of cell.
2. Voltaic (Galvanic) Cells – Spontaneous Reactions
These cells produce electrical energy from a redox reaction that happens without any external energy needed (spontaneous chemical reaction, -ΔG).
- Electrons flow through an external wire from the anode to the cathode.
- The Anode (where oxidation occurs) is negatively charged.
- The Cathode (where reduction occurs) is positively charged.
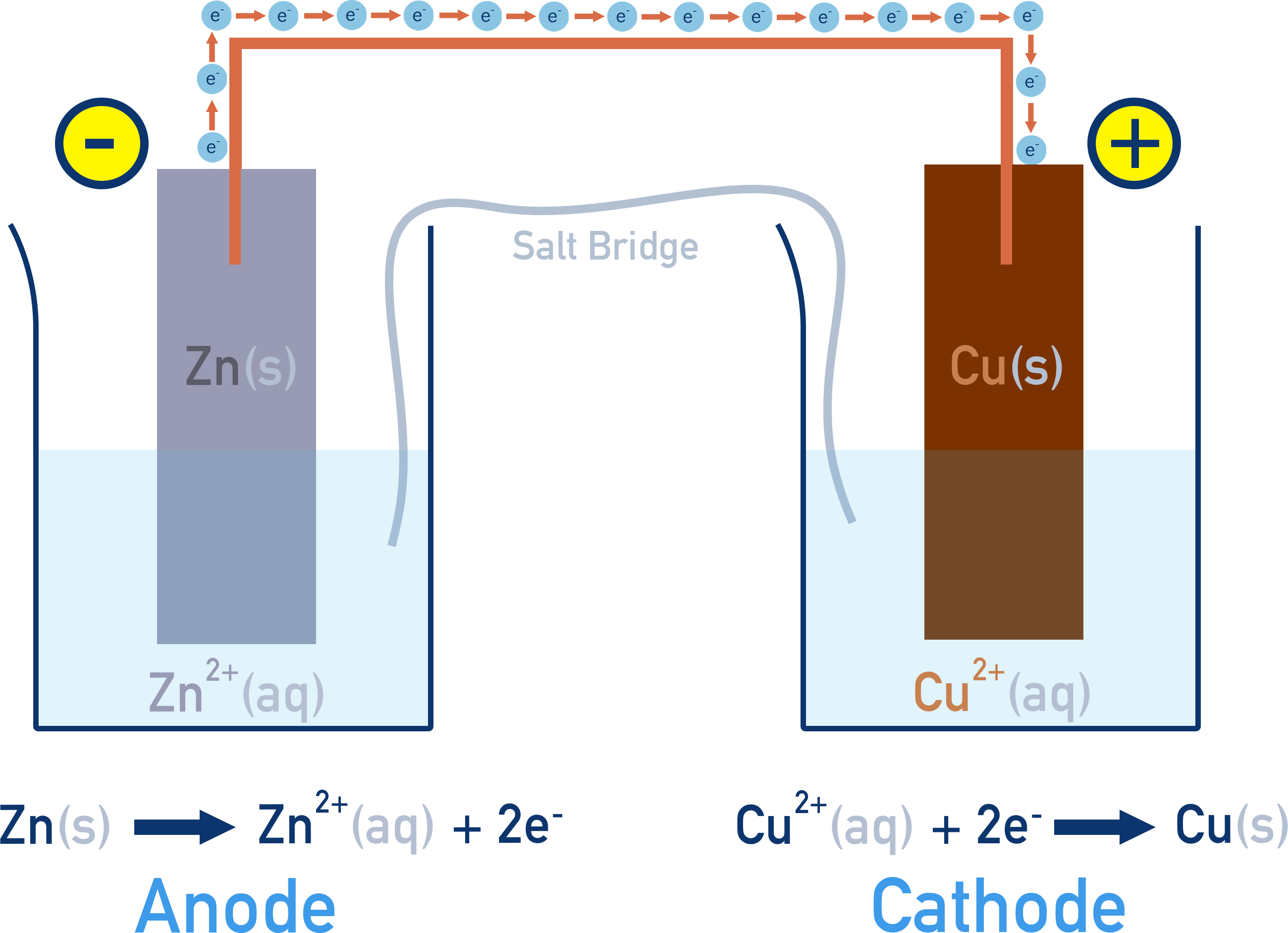
Example: Zinc–copper cell
3. Electrolytic Cells – Non-Spontaneous Reactions
Electrolytic cells use electrical energy to drive a chemical change for Non-spontaneous reactions (+ΔG). Electrons still flow from anode to cathode, but energy is supplied externally.
- The Anode (where oxidation occurs) is positively charged.
- The Cathode (where reduction occurs) is negatively charged.
- Anode often loses mass (oxidation). Cathode often gains mass (reduction).
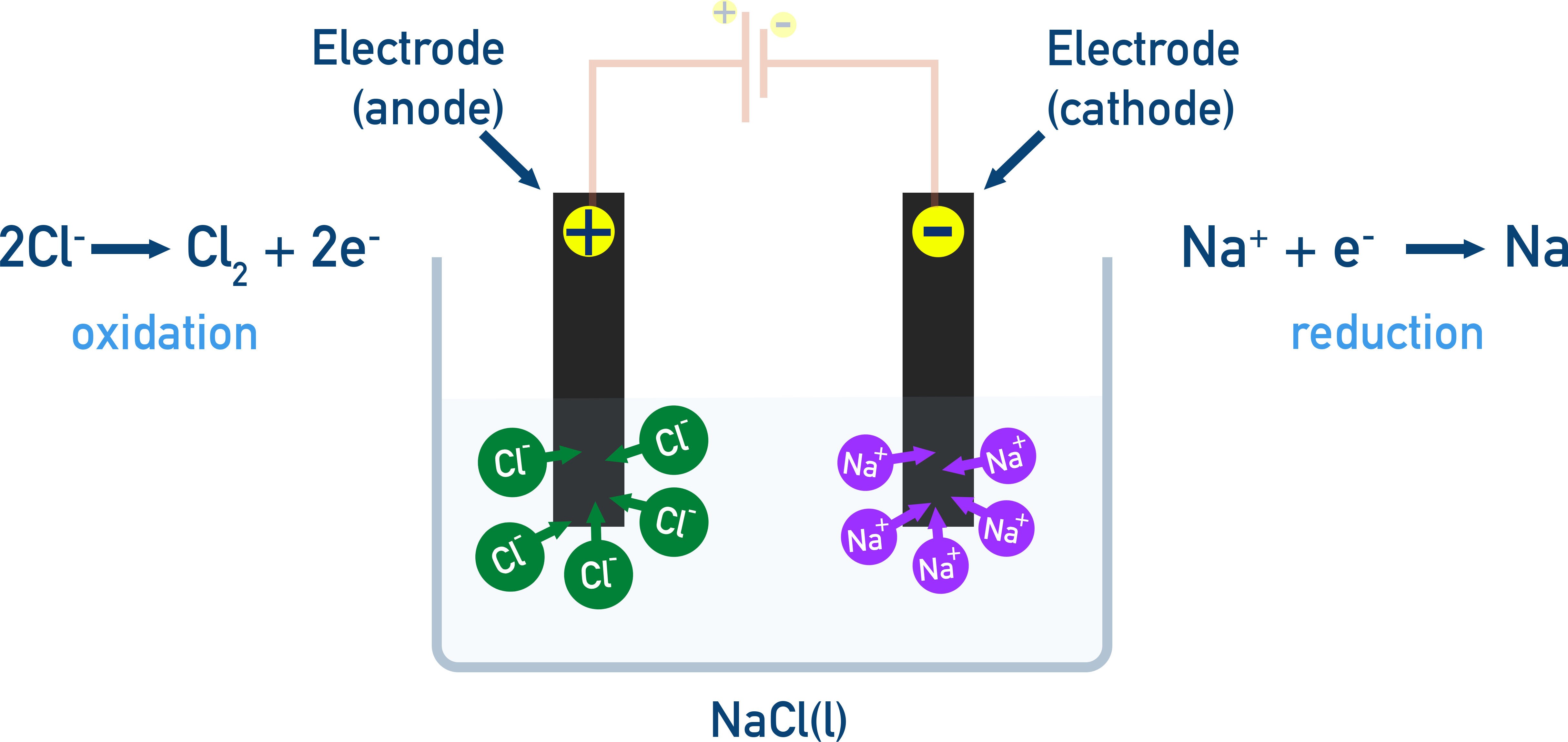
Example: Electrolysis of molten NaCl

Always remember: oxidation happens at the anode, and reduction happens at the cathode. Don't memorise based on positive or negative charges — those switch between voltaic and electrolytic cells. Focus on the reaction type instead: it's consistent every time.
Galvanic (Voltaic) cells in more detail
What exactly is a Voltaic Cell?
A voltaic (or galvanic) cell is a type of electrochemical cell in which a spontaneous redox reaction generates an electric current. A simple voltaic cell can be constructed using two half-cells connected by a salt bridge and an external wire.
Half-Cells
Each half-cell consists of:
- A metal electrode (solid metal)
- An electrolyte (a solution containing ions of that metal)
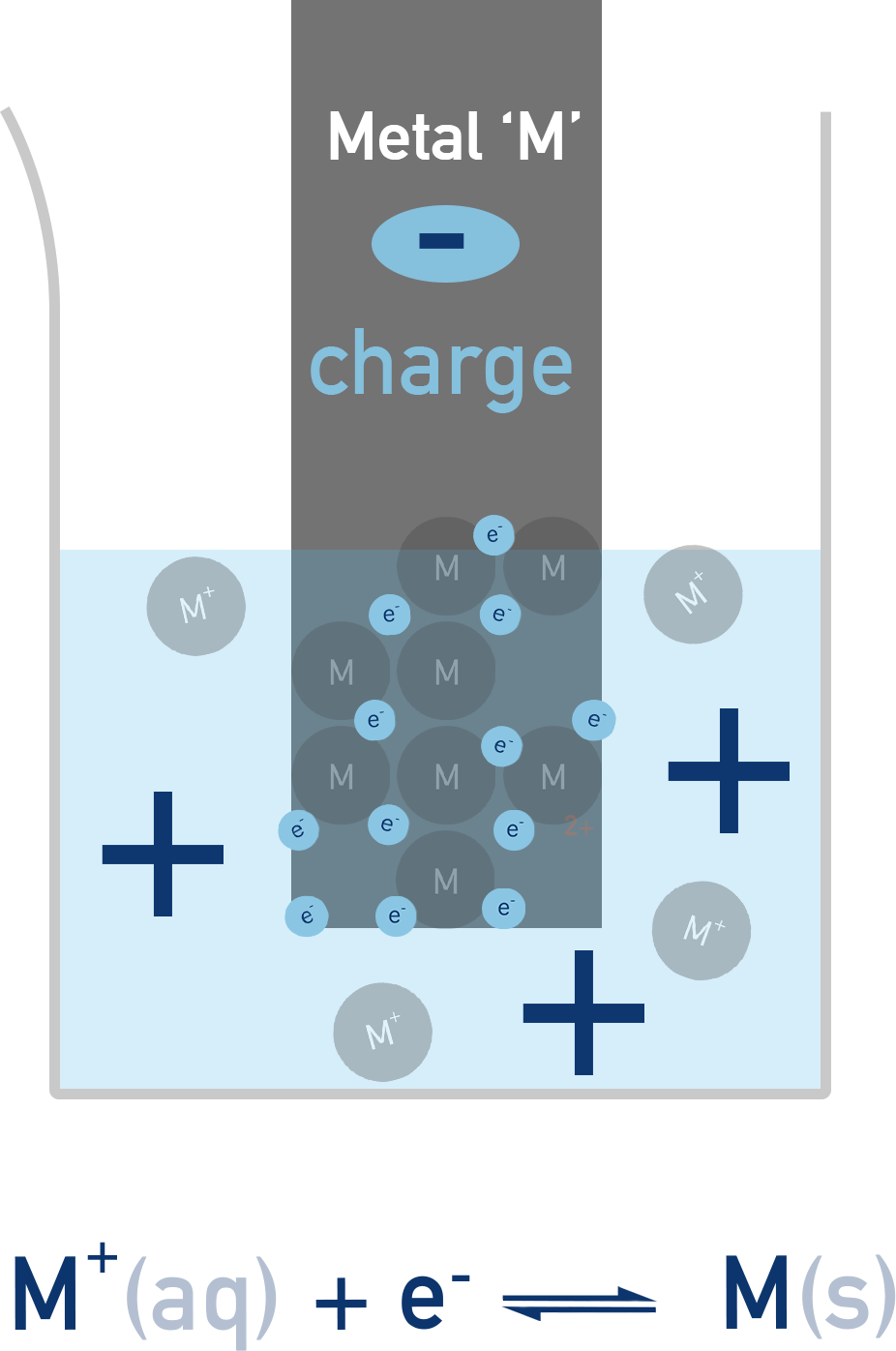
A redox equilibrium is established between the metal atoms and their ions:
Mn+(aq) + n e− ⇌ M(s)
This sets up a potential difference between the metal and the solution. The position of equilibrium – and hence the total charge or ‘potential’ of the electrode – depends on how readily the metal loses or gains electrons. The electrode potential of a half-cell cannot be measured directly, but we can compare it to a standard reference (like the standard hydrogen electrode) to determine its relative value.
Components of a Voltaic Cell
- Anode: site of oxidation
- Metal loses electrons and enters solution as ions
- Electrons flow away from the anode
- Cathode: site of reduction
- Metal ions in solution gain electrons and are deposited as metal
- Electrons flow into the cathode
- Electron flow: Anode → external circuit → Cathode
Salt Bridge Function
- The salt bridge allows ion exchange between half-cells.
- Prevents charge buildup by allowing:
- Cations to migrate toward the cathode
- Anions to migrate toward the anode
- Common salt bridge solution: KNO3 or Na2SO4
How a simple cell works: Zn–Cu Voltaic Cell
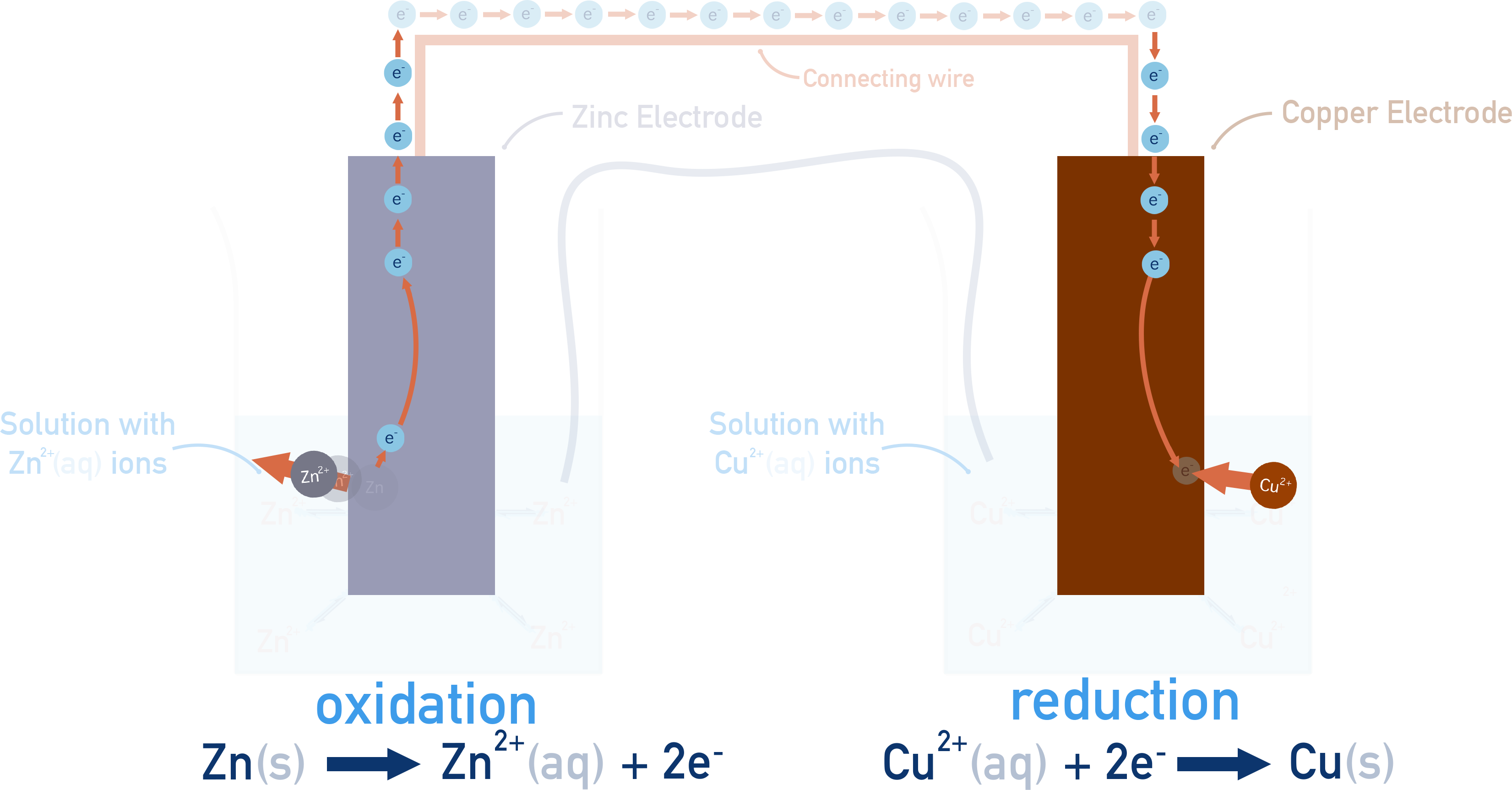
At the anode, zinc metal is oxidised: Zn(s) → Zn2+(aq) + 2e−
The released electrons travel through the external circuit to the cathode, where Cu2+ ions are reduced: Cu2+(aq) + 2e− → Cu(s)
As the reaction proceeds, Zn metal is gradually consumed, and solid copper builds up on the cathode. Electron flow from anode to cathode continues as long as zinc is available to oxidise and Cu2+ ions remain to be reduced. The salt bridge maintains charge balance by allowing ions to move between the two half-cells. This prevents charge buildup, which would otherwise stop the redox reactions from continuing.
Cell Notation
In electrochemistry, cells are written using a shorthand:
- A single vertical line (|) separates different phases (solid, liquid, aqueous).
- A double line (||) represents the salt bridge.
- The anode (oxidation) is written on the left, and the cathode (reduction) on the right.
For example: Write the conventional cell notation for an electrochemical cell made from two half cells made up of the following:
Zn2+(aq) + 2e− ⇌ Zn(s) Cu2+(aq) + 2e− ⇌ Cu(s)
We’ve already established (see above) Cu2+/Cu is the cathode, where reduction happens. Cu2+ will be reduced to Cu. Equally, Zn2+/Zn is the anode, where oxidation happens, Zn will be oxidised to Zn2+. Anode is written on the left with the Zn(s) and Zn2+(aq) separated by a vertical line as they are in different phases. Cathode is written on the right with the Cu2+(aq) and Cu(s) again separated by a vertical line.
Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s)
This shows that electrons flow from zinc (which is oxidised) to copper (which is reduced).
Electrolysis cells in more detail
What Is an Electrolytic Cell?
An electrolytic cell is made up of two electrodes placed into an electrolyte and connected to a DC power source.
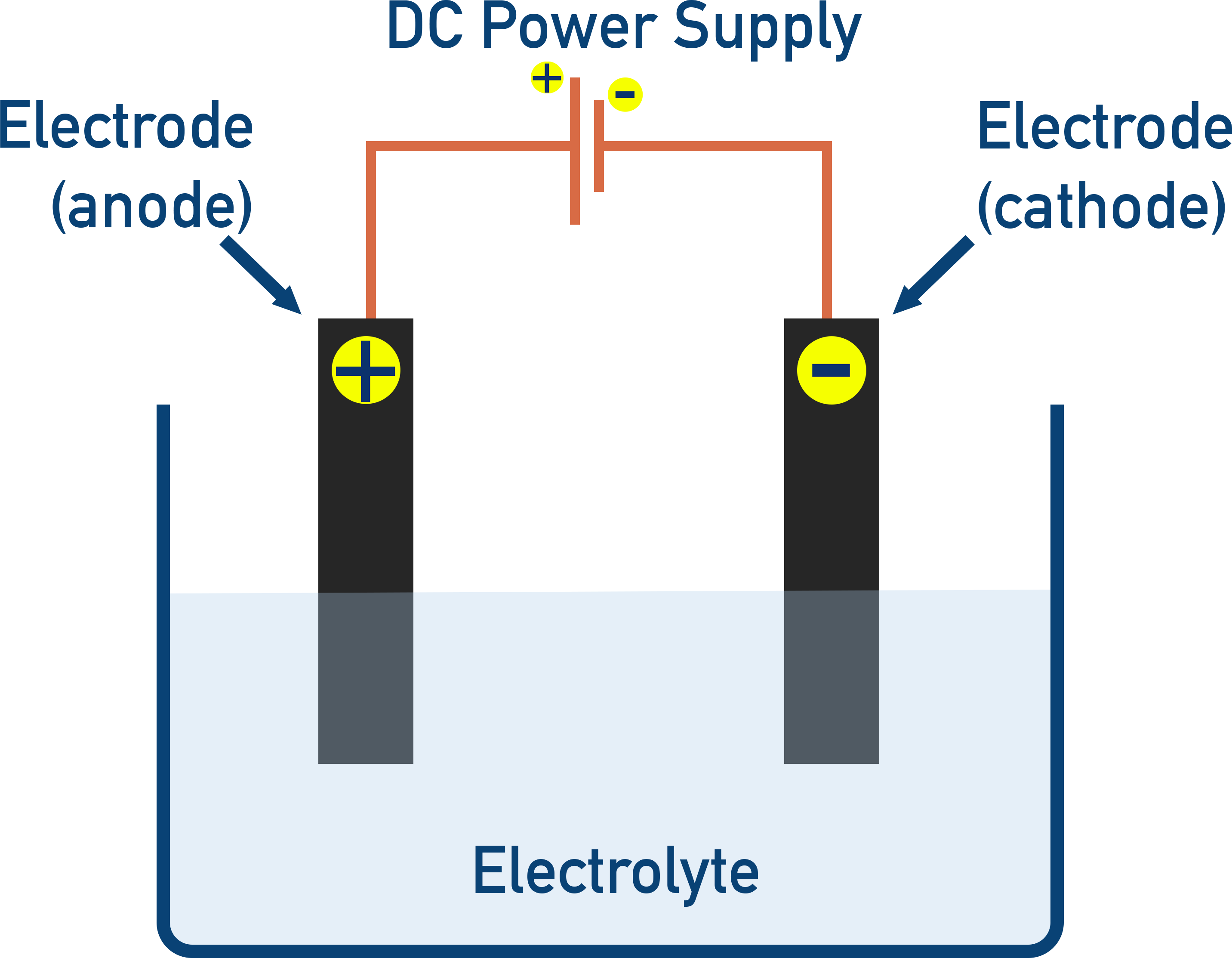
Electrical energy is used to force a non-spontaneous redox reaction to occur.
How does Electrolysis Work?
An electric current is passed through a liquid or molten ionic substance (the electrolyte), which contains free ions that can move and carry charge. Electrons flow from the DC power supply to the cathode. Positive ions migrate to the cathode to gain electrons (reduction), while negative ions move to the anode to lose electrons (oxidation), which return to the positive terminal of the power supply.
- Key point
- The cathode is the negative electrode, where reduction (gain of electrons) happens.
- The anode is the positive electrode, where oxidation (loss of electrons) happens.

I'll repeat what I said earlier - you should always remember the cathode is where reduction takes place and anode where oxidation takes place to avoid any confusion. This is because in voltaic cells the cathode is positively charged and the anode is negatively charged (the opposite way round to electrolysis) however still the cathode is where reduction takes place and the anode where oxidation takes place. If you remember cathode = reduction and anode = oxidation, you will always be correct, regardless of whether the question is about electrolysis or voltaic cells.
Predicting Products of Molten Electrolytes
We can predict the products formed at each electrode based on the type of electrolyte being used. The ionic compound is melted to a liquid state, and only the cations and anions of the compound are present:
- Cation is reduced at the cathode.
- Anion is oxidised at the anode.
For Example: Electrolysis of molten NaCl

Aqueous Electrolytes
The ionic compound is dissolved in water and is aqueous (aq). H+(aq) and OH−(aq) ions from water are also present due to the natural ionisation of water
- 2H2O + 2e− → H2 + 2OH− and
- 2H2O → O2 + 4H+ + 4e−
As a result, water may compete with the ions from the compound at the electrodes for oxidation and reduction. We can use standard electrode potentials (E° values - see Topic 9.9) or reactivity trends to predict which species is discharged.
- At the cathode:
- H+(aq) ions are reduced to H2(g) if the metal is more reactive than hydrogen (lower E° value).
- The metal ion is reduced if it is less reactive than hydrogen (higher E° value).
- At the anode:
- Generally, OH−(aq) is oxidised to form O2 gas.
- If halide ions (e.g. Cl−, Br−) are present, they are oxidised instead.
- Note: Ions like SO42− and NO3− do not get oxidised.
Example: Electrolysis of aqueous NaCl
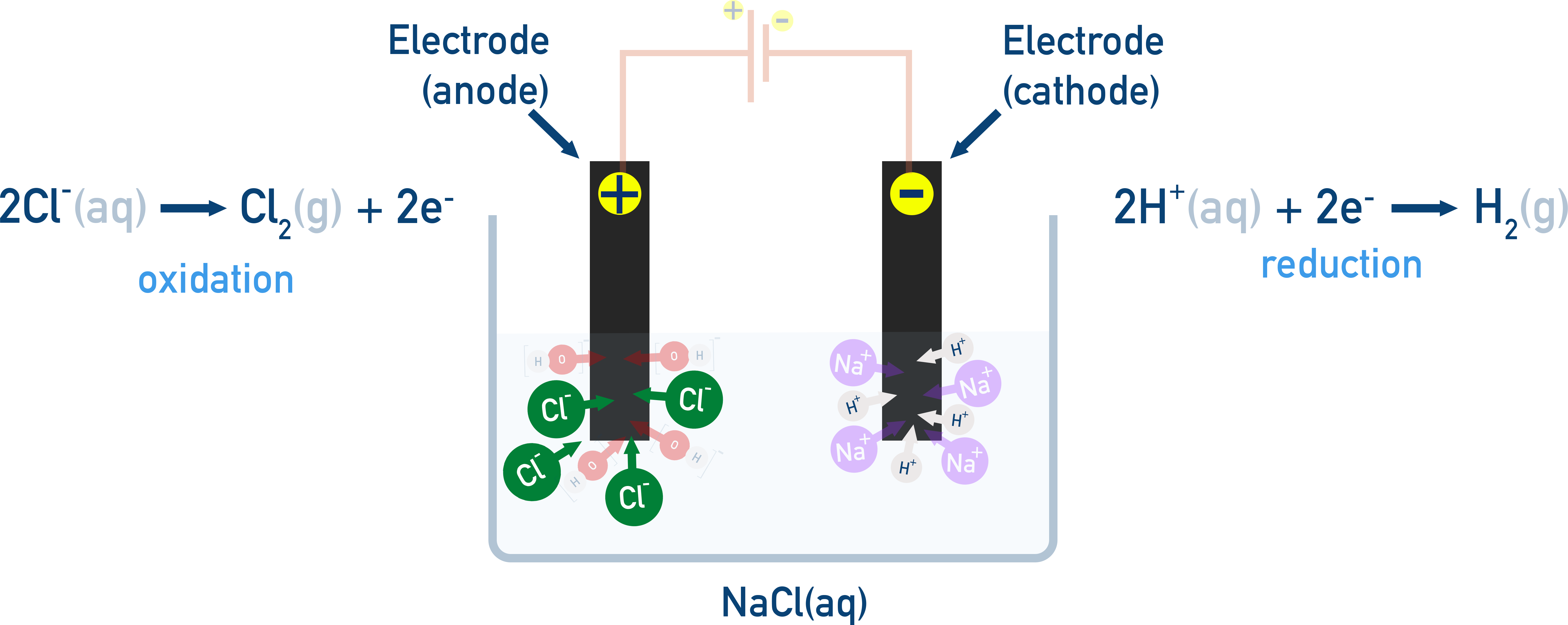
This system contains:
Na+ and H2O (possible reduction at the cathode)
Cl− and H2O (possible oxidation at the anode)
At the Cathode (Reduction) – Competing species:
Na+ + e− → Na (E° = −2.71 V)
2H2O + 2e− → H2 + 2OH− (E° = −0.83 V)
Water is reduced, not sodium (because −0.83 V is more positive):
Cathode reaction: 2H2O + 2e− → H2 + 2OH−
At the Anode (Oxidation) – Competing species:
2Cl− → Cl2 + 2e− (E° = +1.36 V)
2H2O → O2 + 4H+ + 4e− (E° = +1.23 V)
Even though water has a slightly lower reduction potential, Cl− is preferentially oxidised in concentrated solutions of NaCl (this is due to other factors such as kinetics):
Anode reaction: 2Cl− → Cl2 + 2e−
Another Example: Electrolysis of Aqueous CuSO4
CuSO4(aq) contains:
- Cu2+ and SO42− from the salt
- H2O, which contributes H+ and OH− ions
Cathode: Possible Reductions
Compare the reduction potentials:
Cu2+ + 2e− → Cu(s) E° = +0.34 V
2H2O + 2e− → H2 + 2OH− E° = −0.83 V
Copper is reduced, because it has a much more positive E° value than hydrogen gas.
Cathode reaction: Cu2+(aq) + 2e− → Cu(s) (Copper metal is deposited on the electrode)
We compare the reverse of these reduction potentials:
S2O82− + 2e− → 2SO42− E° = +2.01 V
O2 + 4H+ + 4e− → 2H2O E° = +1.23 V
(Note: Halide ions like Cl− are not present.)
Since oxidation is the reverse of reduction, the species with the lower E° for reduction is easier to oxidise. Water (E° = +1.23 V) is oxidised more readily than S2O82−.
Anode reaction: 2H2O → O2(g) + 4H+ + 4e−
Concentration matters:
If halide concentration is very low (e.g., dilute NaCl), OH− from water may be oxidised instead.
If the metal ion concentration is very low, hydrogen gas may form instead of metal at the cathode.
