Calculating the Equilibrium Constant
Quick Notes
- Kc uses equilibrium concentrations (mol dm−3) and Kp uses equilibrium partial pressures.
- Both are calculated using equilibrium data (equilibrium concentrations for Kc or partial pressures for Kp).
- Balanced chemical equations can be used to construct the correct equilibrium expression.
- Only include species with variable concentration (don't include pure solids and liquids).
- Units of K vary depending on the reaction and should be consistent with the expression.
Recap — What is the Equilibrium Constant?
The equilibrium constant measures the ratio of products to reactants at equilibrium for a given reaction.

The Kc expression (concentration):
![Kc expression: Kc equals [C]^c [D]^d divided by [A]^a [B]^b](images/kcexpression2.png)
The Kp expression (partial pressure):
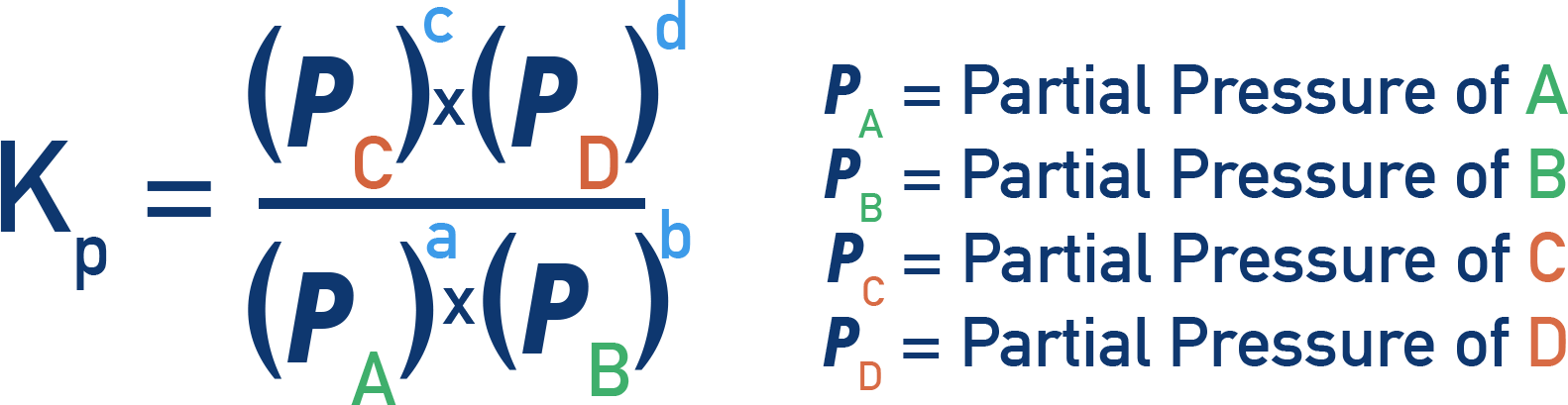
Steps to Calculate Kc or Kp
- Write the balanced equation. This gives you the molar ratios and sets the exponents (powers) for the K expression.
- Construct the K expression. Use concentrations for Kc, partial pressures for Kp.
- Insert equilibrium values. Read them from the question or determine them via an ICE table.
- Calculate. Apply exponents first; then multiply/divide to find K (and units if required).
Reaction: CH3COOH + C2H5OH ⇌ CH3COOC2H5 + H2O
Equilibrium concentrations: [CH3COOH] = 0.20, [C2H5OH] = 0.20, [CH3COOC2H5] = 0.40, [H2O] = 0.40 (mol dm−3)
- Kc = [ester][water] / ([acid][alcohol])
- Kc = (0.40 × 0.40) / (0.20 × 0.20) = 4.0
Since Kc > 1, equilibrium favours the products. This means in the equilibrium mixture there is a higher concentration of products (ester and water) compared to reactants.

Remember concentrations in the Kc expression are the concentrations of everything at equilibrium. Make sure you read questions carefully and, if needed, calculate equilibrium concentrations from given data (see below).
Find Kp for the following system
Reaction: N2O4(g) ⇌ 2 NO2(g)Given: total pressure = 400 kPa; n(N2O4) = 0.40, n(NO2) = 0.60.
- Mole fractions: χ(N2O4) = 0.40; χ(NO2) = 0.60.
- Partial pressures: P(N2O4) = 0.40×400 = 160 kPa; P(NO2) = 0.60×400 = 240 kPa.
- Kp = (P(NO2))2 / P(N2O4) = (240)2 / 160 = 360 kPa.
ICE Example
Calculate the equilibrium concentration of H₂ for the following.
Reaction: H2(g) + I2(g) ⇌ 2HI(g), K = 50.0 at 298 KAt equilibrium: [HI] = 0.80 mol dm−3, [I2] = 0.10 mol dm−3. Find [H2].
| Species | Initial | Change | Equilibrium |
|---|---|---|---|
| H2 | a | −0.40 | a − 0.40 |
| I2 | — | −0.40 | 0.10 |
| HI | 0 | +0.80 | 0.80 |
- K = [HI]2 / ([H2][I2])
- 50.0 = (0.80)2 / [(a − 0.40)(0.10)] → 0.10(a − 0.40) = 0.0128
- a − 0.40 = 0.128 → a = 0.528 → [H2] = a − 0.40 = 0.128 mol dm−3.
Approximation Use (K ≪ 1)
If K is very small, then very little product is present at equilibrium. Because of this, we can assume the reactant’s equilibrium concentration is approximately its initial value.
For Example:
Lets take the following equilibrium,
A ⇌ B
The equilibrium constant is K = 1.0 × 10⁻⁵
Initially:
[A] = 0.20 mol dm⁻³
[B] = 0 mol dm⁻³
Estimate the equilibrium concentration of B.
-
Step 1: ICE table (assume small change)
Let the change in A be –x and the change in B be +x:Species Initial Change Equilibrium A 0.20 –x 0.20 – x B 0 +x x -
Step 2: Write expression for K
K = [B] / [A] = x / (0.20 – x)
Since K is very small, we assume that x is small and so 0.20 – x ≈ 0.20
So:
1.0 × 10⁻⁵ = x / 0.20
x = 0.20 × 1.0 × 10⁻⁵ = 2.0 × 10⁻⁶ mol dm⁻³
Conclusion:
At equilibrium, only a tiny amount of B is formed, and [A] hardly changes – justifying the approximation.
So:
[A] ≈ 0.20 mol dm⁻³
[B] ≈ 2.0 × 10⁻⁶ mol dm⁻³
Summary
- Use measured equilibrium concentrations/pressures to compute K.
- Follow the law of mass action; apply exponents from the balanced equation.
- K indicates how far a reaction proceeds toward products.
- Kc for concentrations; Kp for partial pressures.
