Reaction Mechanisms and Rate-Determining Step HL Only
Quick Notes
- Reactions often occur in a series of elementary steps, not all at once.
- The slowest step is the rate-determining step (RDS).
- Reaction intermediates are formed in one step and used in a later one.
- Transition states are high-energy states between reactants and products that don't exist for a measurable amount of time.
- Energy profiles can show multiple steps, each with a peak (transition state).
- Mechanisms must be consistent with:
- Kinetic data (rate equation)
- Stoichiometry (overall balanced equation)
- The RDS is not always the first step.
Full Notes
Many chemical reactions don’t happen in one step, they occur through a sequence of elementary steps:
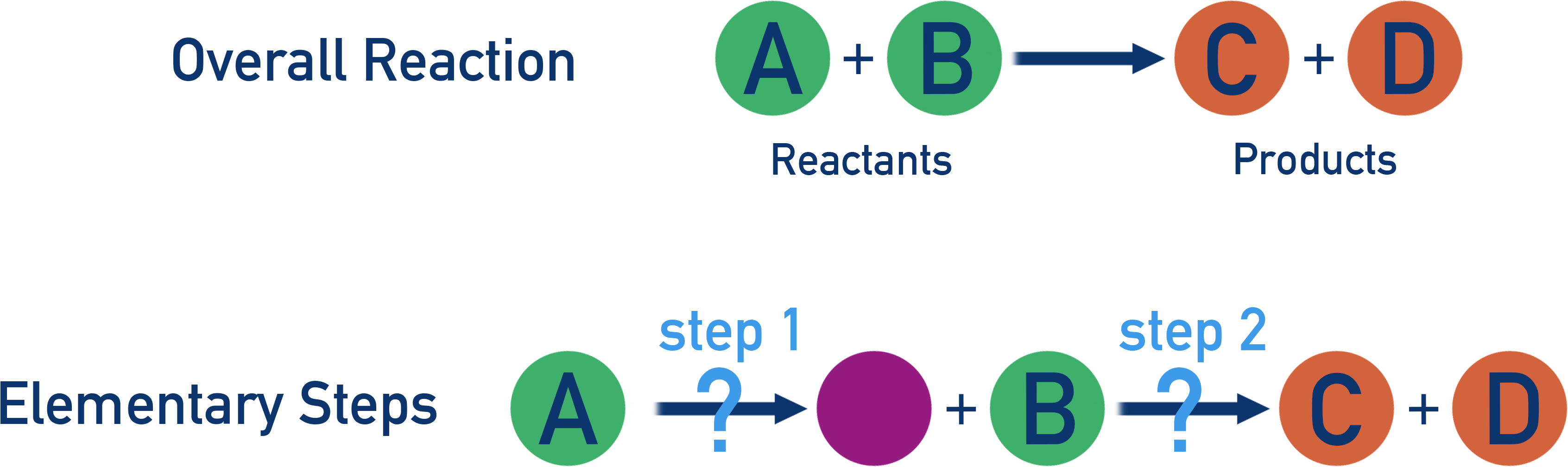
Each step involves the breaking or forming of a small number of bonds. The overall reaction is the sum of all elementary steps that occur.
How the steps link together is referred to as the ‘reaction mechanism’.
Rate-Determining Step (RDS)
Each step in a multi-step reaction will have its own rate. As a result, it is the slowest elementary step in a mechanism that limits the overall reaction rate.
This slowest step is called the rate determining step (RDS).
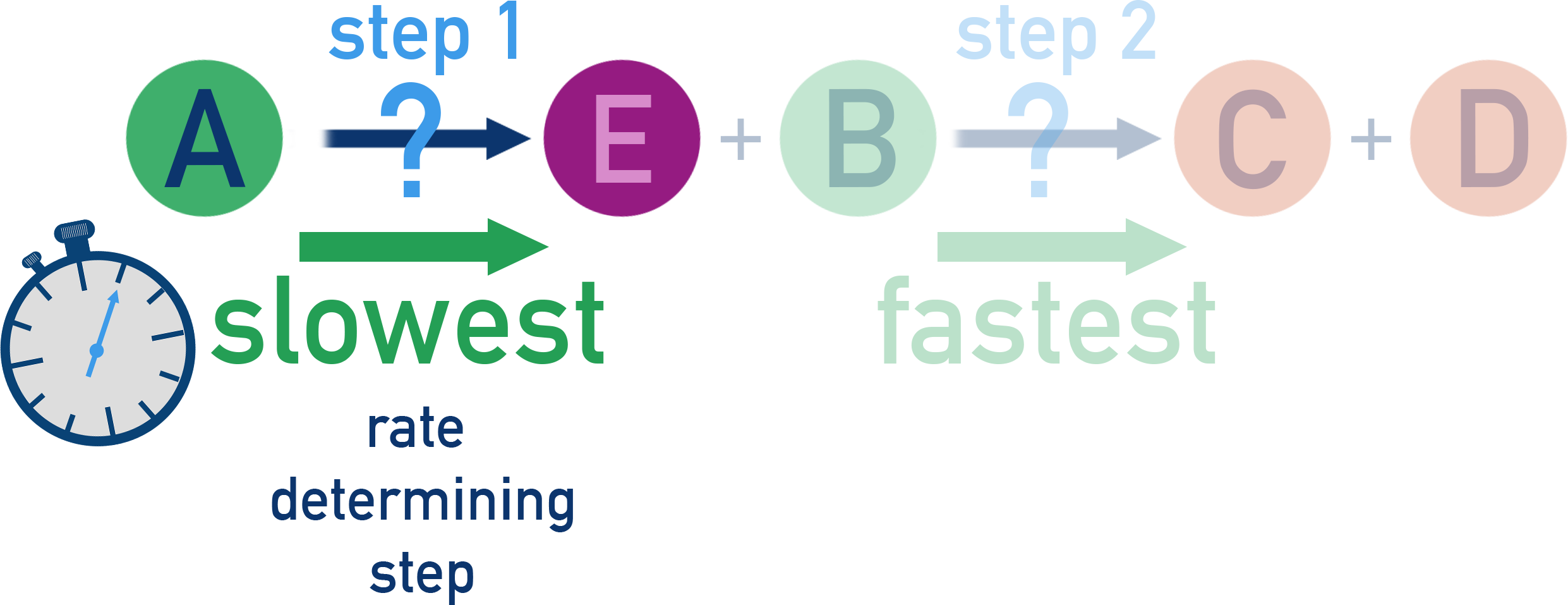
A reaction can only go as fast as the rate determining step in its mechanism.
The rate determining step also determines the rate equation (see 2.2.9 for more detail).
A species will only appear in the rate equation if it is part of (or influences) the RDS.
Intermediates vs. Transition States
Understanding the difference between intermediates and transition states is crucial for interpreting reaction mechanisms and energy profiles.
Intermediates:
Intermediates are formed during a reaction and exist for a measurable amount of time before turning into products. They do not appear in the overall equation.
Transition states:
Transition states are high-energy, unstable configurations that arise during bond breaking/forming. They don’t actually exist for any measurable amount of time - a bit like imagining a ball as its thrown up in the air, at its maximum height.
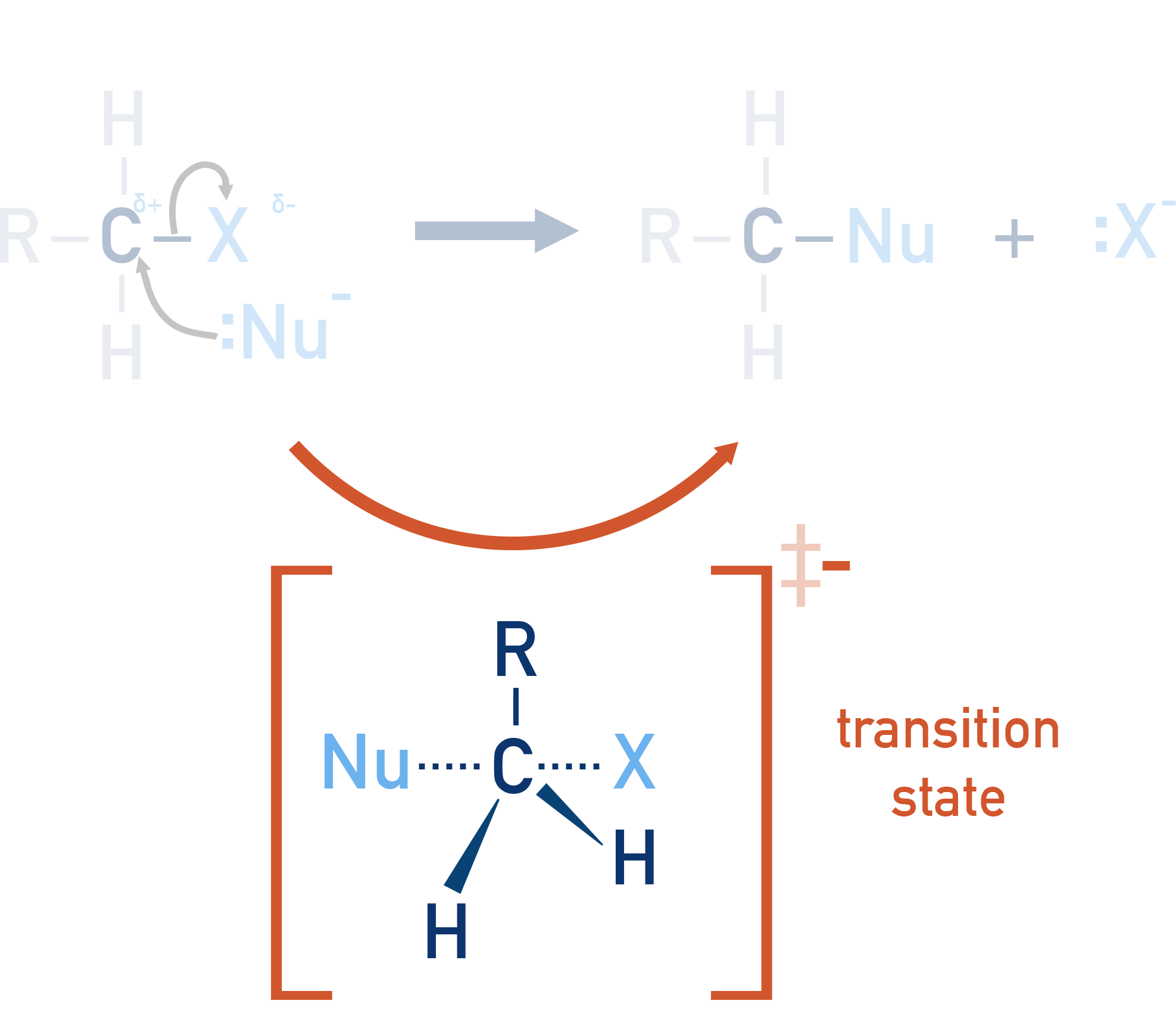
Transition states are represented as peaks on an energy profile (see below).

Key distinction: Intermediates are real, though short-lived whereas transition states are theoretical high energy configurations - they can't be isolated.
Energy Profile with Multiple Steps
A multi-step reaction will have multiple peaks in its energy profile diagram:
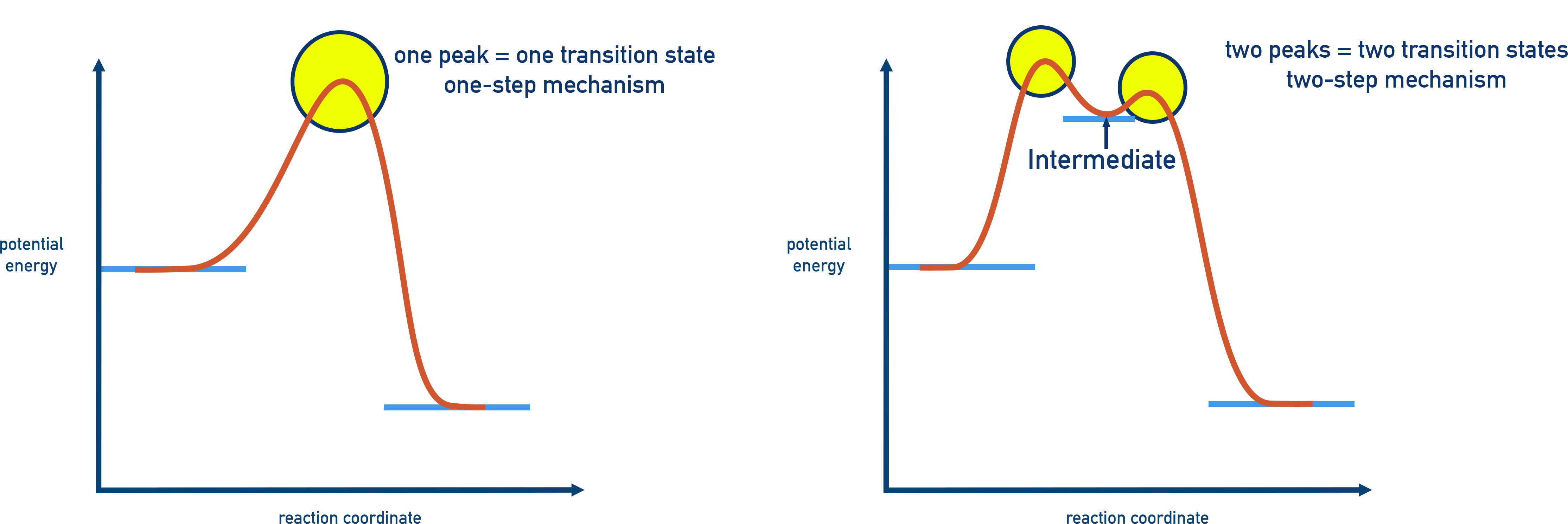
- Each peak = transition state.
- Each valley = intermediate.
The highest peak corresponds to the rate determining step (RDS), as it has the largest energy barrier (Ea).
Matching Mechanisms to Data
Mechanisms are predicted based on experimental data and a proposed mechanism must:
- Match the overall balanced equation (stoichiometry).
- Be consistent with the rate equation (derived from experimental kinetics).

Remember that mechanisms are proposed using experimental kinetic and stoichiometric data. They are only predictions, there may be more than one possible mechanism for a given reaction.
Rate-Determining Step (RDS) Beyond Step One
In many mechanisms, the slowest step (rate-determining step) occurs after the first step, often involving an intermediate formed earlier.
Example: Reaction of NO2 and CO
Overall equation:
NO2 + CO → NO + CO2
Mechanism:
- NO2 + NO2 → NO + NO3 (fast)
- NO3 + CO → NO2 + CO2 (slow, rate-determining)
The second step is the RDS, even though it's not first. The intermediate NO3 is formed in step 1 and consumed in step 2. Since step 2 is the slow step, the rate depends on the concentrations of the reactants involved in (or before) that step.
Summary
- Reactions occur in elementary steps and the slowest step is the rate determining step, RDS.
- The RDS determines the rate law.
- Intermediates are short-lived species and are real.
- Transition states are theoretical peaks.
- Mechanisms must agree with kinetics and stoichiometry.
Which mechanism in the hydrolysis of halogenoalkanes involves an intermediate?
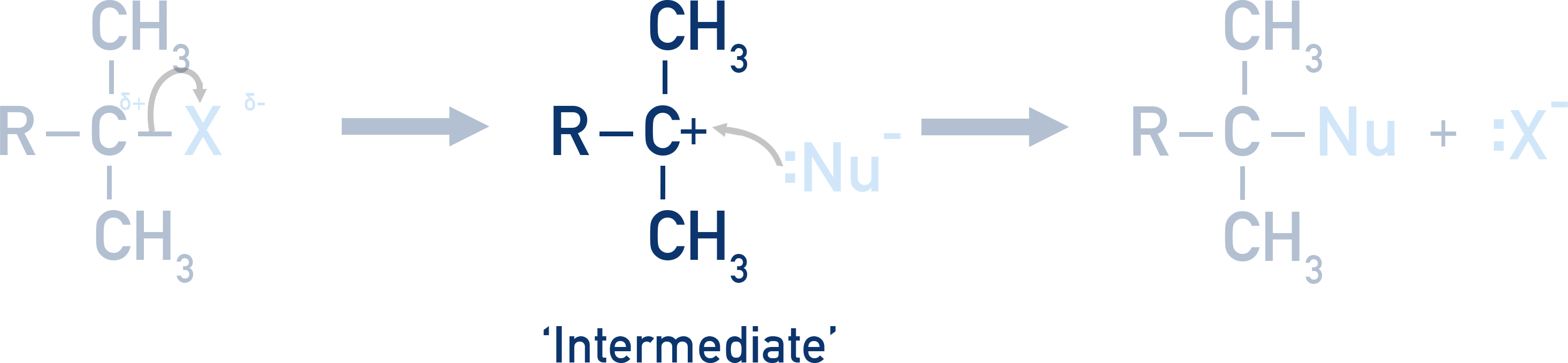
The SN1 mechanism (unimolecular nucleophilic substitution) involves a carbocation intermediate.
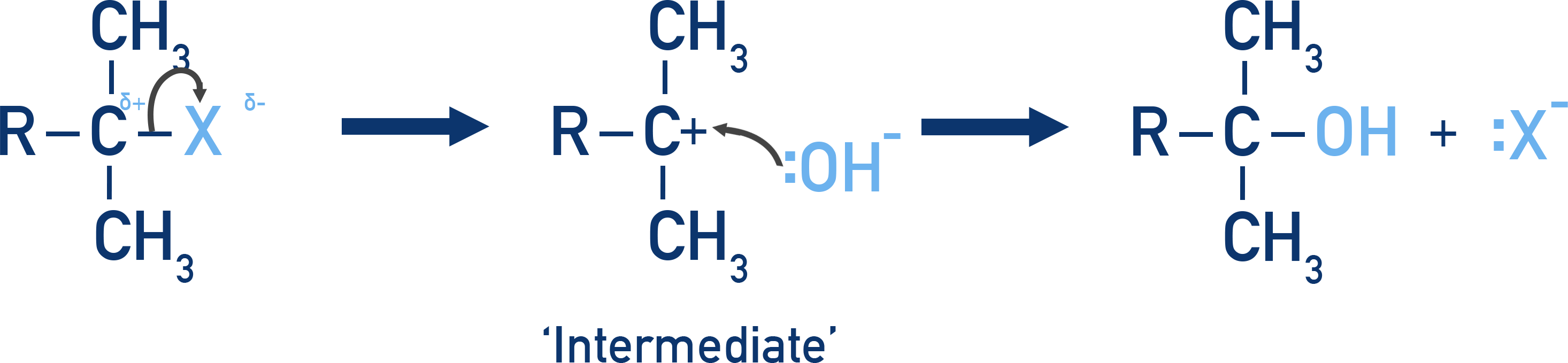
In this mechanism, the halogenoalkane first loses the halide ion in a slow step, forming a positively charged carbocation. This is followed by a fast attack by a nucleophile (e.g. water or OH−) on the intermediate. SN1 is typical for tertiary halogenoalkanes, where the carbocation is stabilized by surrounding alkyl groups.
The SN2 mechanism (bimolecular) occurs in only one step, with no intermediate formed.

