Successive Ionization Energies and Electron Configuration HL Only
Quick Notes:
- Successive ionization energies refer to the energy required to remove each electron one at a time from the same atom.
- First Ionisation Energy (IE1): energy to remove the first electron
- Second Ionisation Energy (IE2): energy to remove the second electron
- Third Ionisation Energy (IE3): third, and so on…
- A large jump in ionization energy shows that an electron is being removed from a new inner energy level.
- The number of electrons before the big jump indicates the number of valence electrons and this helps determine the group number.
Full Notes:
What Are Successive Ionization Energies?
Atoms can lose more than one electron, but each additional electron is harder to remove than the last.
This is because the positive charge increases each time an electron is removed and there are fewer electrons left to repel each other.
As a result, successive ionisation energies (IEs) increase.
Example: Aluminium
IE1 Al(g) → Al+(g) + e⁻ = 578 kJ mol⁻¹
IE2 Al+(g) → Al2+(g) + e⁻ = 1817 kJ mol⁻¹
IE3 Al2+(g) → Al3+(g) + e⁻ = 2745 kJ mol⁻¹
IE4 Al3+(g) → Al4+(g) + e⁻ = 11,580 kJ mol⁻¹ ← large jump here
Interpreting the Data
The key to analysing successive ionization energies is looking for big jumps.
| Ionisation Number | Ionisation Energy (kJ/mol) |
|---|---|
| 1st | 577 |
| 2nd | 1816 |
| 3rd | 2745 |
| 4th | 11578 |
| 5th | 14842 |
Small, steady increases between successive values = valence electrons.
For example in the table above, 1st, 2nd and 3rd ionization energies are increasing steadily - meaning they are likely all removing valence (outermost) electrons.
A large jump = removing an electron from a lower energy level (closer to the nucleus and more strongly held).
For example in the table above, there is a big jump from the 3rd to 4th ionization energy, meaning the 4th is likely removing an electron from a new energy level.
How to Deduce the Group
Count the number of electrons removed before the big jump. That number = the number of valence electrons = the group number in the periodic table.
Example:If there's a big jump between IE3 and IE4 then the element has 3 valence electrons and it's in Group 13 (III).
Visualising with Graphs
Successive ionization energies are often shown as bar graphs.
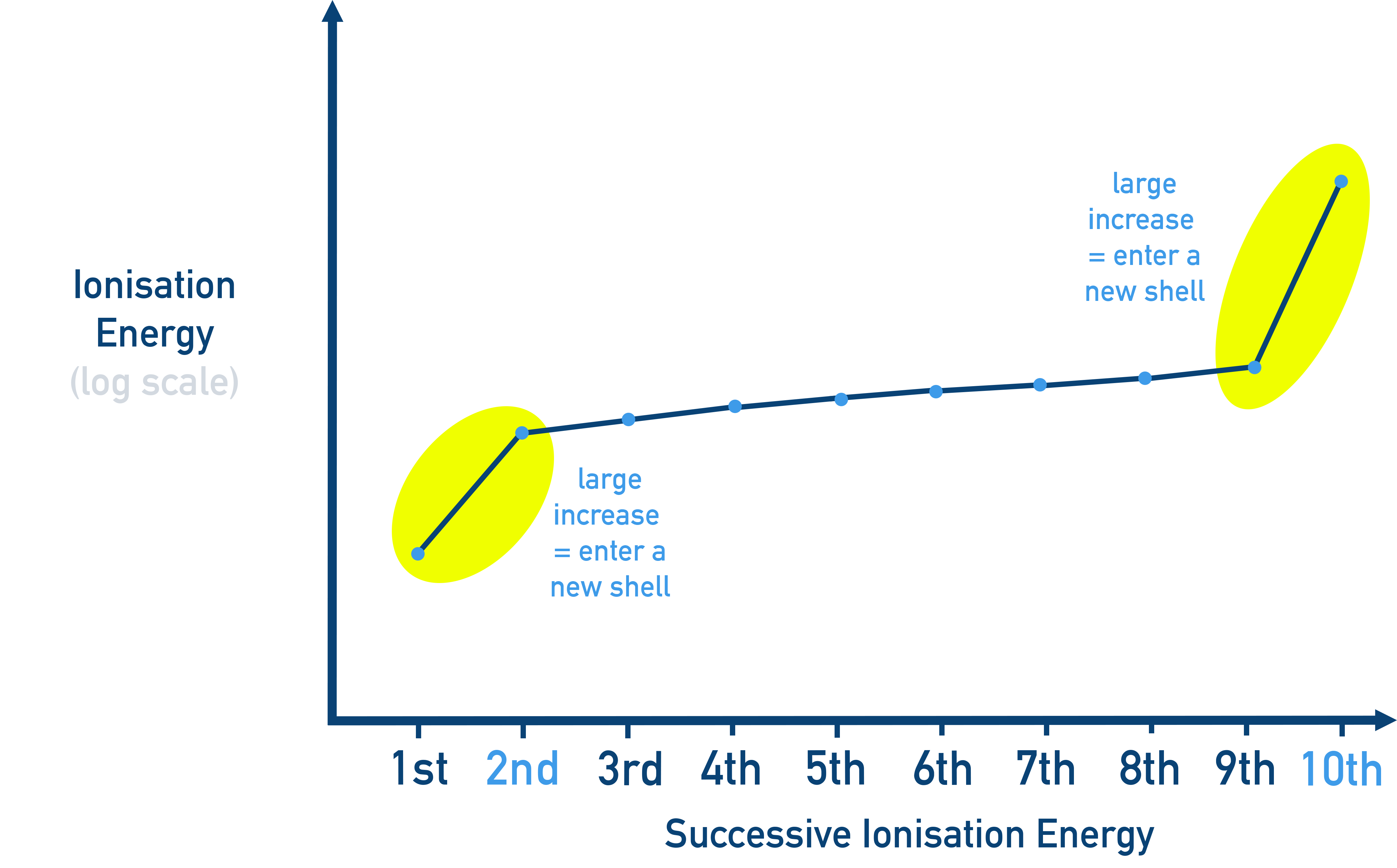
A sharp rise on the graph indicates an electron is being removed from a new, lower shell.
Summary
- Successive ionization energies increase as more electrons are removed.
- Large jumps indicate removal of an electron from a new inner energy level.
- Counting electrons before the big jump reveals the number of valence electrons.
- Valence electron count links to group number in the periodic table.
Linked Questions
How do patterns of successive ionisation energies of transition elements help to explain the variable oxidation states of these elements?
Successive ionisation energy (IE) values of transition elements show relatively small increases between the removal of 4s and 3d electrons. This indicates that both types of electrons are close in energy and can be lost in chemical reactions. As a result, transition metals can form ions with different numbers of electrons removed, leading to multiple oxidation states. For example, iron can form Fe²⁺ by losing two 4s electrons or Fe³⁺ by losing an additional 3d electron. The gradual rise in successive IEs across the series reflects increasing nuclear charge and decreasing atomic radius but still allows flexibility in oxidation number.
