Oxidation and Reduction
Quick Notes
- Oxidation: loss of electrons, increase in oxidation number, or gain of oxygen / loss of hydrogen.
- Reduction: gain of electrons, decrease in oxidation number, or loss of oxygen / gain of hydrogen.
- Oxidation state: shows how many electrons an atom has lost or gained.
- Oxidizing agent: causes oxidation (is reduced).
- Reducing agent: causes reduction (is oxidized).
- Use oxidation numbers to:
- Identify redox processes
- Name compounds with Roman numerals (e.g. iron(III) chloride)
- Transition metals and non-metals often show variable oxidation states.
Full Notes
Definitions of Oxidation and Reduction
Oxidation and reduction can be define in several ways - and it depends on the type of reaction being studied as to which are most relevant.
| Definition Type | Oxidation | Reduction |
|---|---|---|
| Electron transfer | Loss of electrons | Gain of electrons |
| Oxidation number | Increase in oxidation number | Decrease in oxidation number |
| Oxygen/hydrogen | Gain of oxygen or loss of hydrogen | Loss of oxygen or gain of hydrogen |
Oxidation State (Oxidation Number)
Oxidation states help track electron transfer in reactions. It is straightforward to see how atoms have lost or gained electrons when ions get formed, however it can be harder to see how atoms have lost or gained electron density when dealing with molecules.
For example, carbon is oxidised to form carbon dioxide when combusted. However, no ions get formed, meaning it isn’t immediately clear how electrons are involved!

To help, we consider each atom to have an ‘imaginary’ charge, described as its oxidation number (or state).
Rules for assigning oxidation states:
- Uncombined elements (e.g., O2, N2, Fe) have an oxidation state of 0.
- Group 1 metals = +1, Group 2 metals = +2.
- Oxygen is –2, except:
- In peroxides (O22−), oxygen is –1.
- With fluorine (OF2), oxygen is +2.
- Hydrogen is +1, except in metal hydrides (e.g., NaH), where it is –1.
- In a neutral compound, the sum of oxidation states = 0.
- In polyatomic ions, the sum of oxidation states = charge of the ion.
Using these rules, we can see how carbon gets oxidised from an oxidation state of 0 in C(s) to +4 in CO2(g).
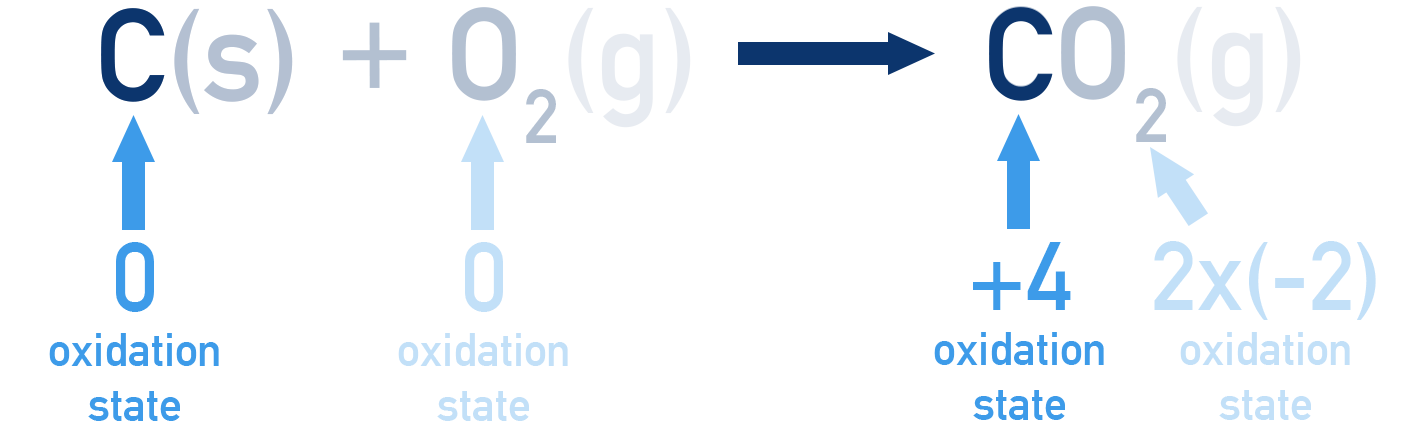
An increase in oxidation number (more positive) means oxidation has occurred.
A decrease in oxidation number (more negative) means reduction has occurred.
Rules for Assigning Oxidation Numbers
| Rule | Example |
|---|---|
| Uncombined elements = 0 | H2, Cl2, Na ⇒ 0 |
| Group 1 metals = +1 | Na+ = +1 |
| Group 2 metals = +2 | Mg2+ = +2 |
| Fluorine = -1 (always) | F in HF = -1 |
| Hydrogen = +1 | H in H2O = +1 |
| Hydrogen = -1 (only in metal hydrides) | H in NaH = -1 |
| Oxygen = -2 | O in H2O = -2 |
| Oxygen = -1 (only in peroxides) | O in H2O2 = -1 |
Example: Assign oxidation states in H2SO4
- H = +1 (2 atoms, total +2).
- O = –2 (4 atoms, total –8).
- Total must be 0, so sulfur = +6.
Roman Numerals in Names
Oxidation numbers are shown in Roman numerals in the names of compounds — particularly for transition metals and other elements with variable oxidation states.
Examples:
- FeCl2 → iron(II) chloride → Fe = +2
- FeCl3 → iron(III) chloride → Fe = +3
- MnO4− → manganate(VII) ion → Mn = +7
Oxidising and Reducing Agents
In any redox reaction, one species donates electrons (reducing agent), and another accepts them (oxidising agent).
- Oxidising agent:
- Accepts electrons (is reduced).
- Causes another species to be oxidised.
- Reducing agent:
- Donates electrons (is oxidised).
- Causes another species to be reduced.
Example: Zn + Cu2+ → Zn2+ + Cu
Zn is oxidised (loses electrons) acts as a reducing agent.
Cu2+ is reduced (gains electrons) acts as an oxidising agent.
Variable Oxidation States
Some elements, particularly the transition metals, can form ions with different charges – meaning they have variable oxidation states.
Some non-metals also show multiple oxidation numbers.
Examples:
- Transition metals: Fe2+ and Fe3+, Mn2+ to Mn7+
- Non-metals: S = –2 in H2S, +6 in H2SO4; N = –3 in NH3, +5 in HNO3
Oxidation Numbers in Compound Names
When an element has more than one possible oxidation state, Roman numerals in names indicate which form is present.
This is especially useful with transition metals and oxyanions.
For Examples
- Iron(II) chloride: FeCl2 (Fe2+)
- Iron(III) chloride: FeCl3 (Fe3+)
- Manganese(VII) oxide: Mn2O7 (Mn7+)
Summary
- Oxidation is loss of electrons or increase in oxidation state and reduction is the opposite.
- Oxidation states track redox changes and appear in systematic naming.
- Oxidising agents gain electrons and reducing agents lose electrons.
- Transition metals and non-metals can show variable oxidation states.
Linked Course Question
What are the advantages and limitations of using oxidation states to track redox changes?
Advantages:
- Simplifies redox reactions and shows clearly which species are oxidised or reduced.
- Can be applied to covalent compounds where ions are not present.
- Helps systematic naming of compounds.
- Aids balancing redox equations in acidic or basic solutions.
Limitations:
- Oxidation states are not always “real” charges, especially in covalent compounds.
- Exceptions exist that can be confusing, such as in peroxides or superoxides.
The surface oxidation of metals is often known as corrosion. What are some of the consequences of this process?
Corrosion is a redox process in which reactive metals are oxidised by oxygen, often in the presence of water, forming metal oxides such as rust in iron.
Consequences:
- Loss of material, reducing strength, durability, and mass.
- Some oxides form protective barriers, e.g. Al2O3, preventing further oxidation.
- Oxidation of transition metals can reduce or destroy catalytic activity.
