Primary (Voltaic) Cells
Quick Notes
- A voltaic (primary) cell converts chemical energy from a spontaneous redox reaction into electrical energy.
- Electrons flow through the external circuit from anode (oxidation) to cathode (reduction).
- Ions move through the salt bridge to maintain electrical neutrality.
- A complete voltaic cell includes:
- Two half-cells (metal/metal ion)
- Anode and cathode
- Salt bridge
- External wire (circuit)
Full Notes
A voltaic (or galvanic) cell is a type of electrochemical cell in which a spontaneous redox reaction generates an electric current.
A voltaic cell can be constructed using two half-cells connected by a salt bridge and an external wire.
Half-Cells
A simple half-cell consists of:
- A metal electrode (solid metal)
- An electrolyte (a solution containing ions of that metal)
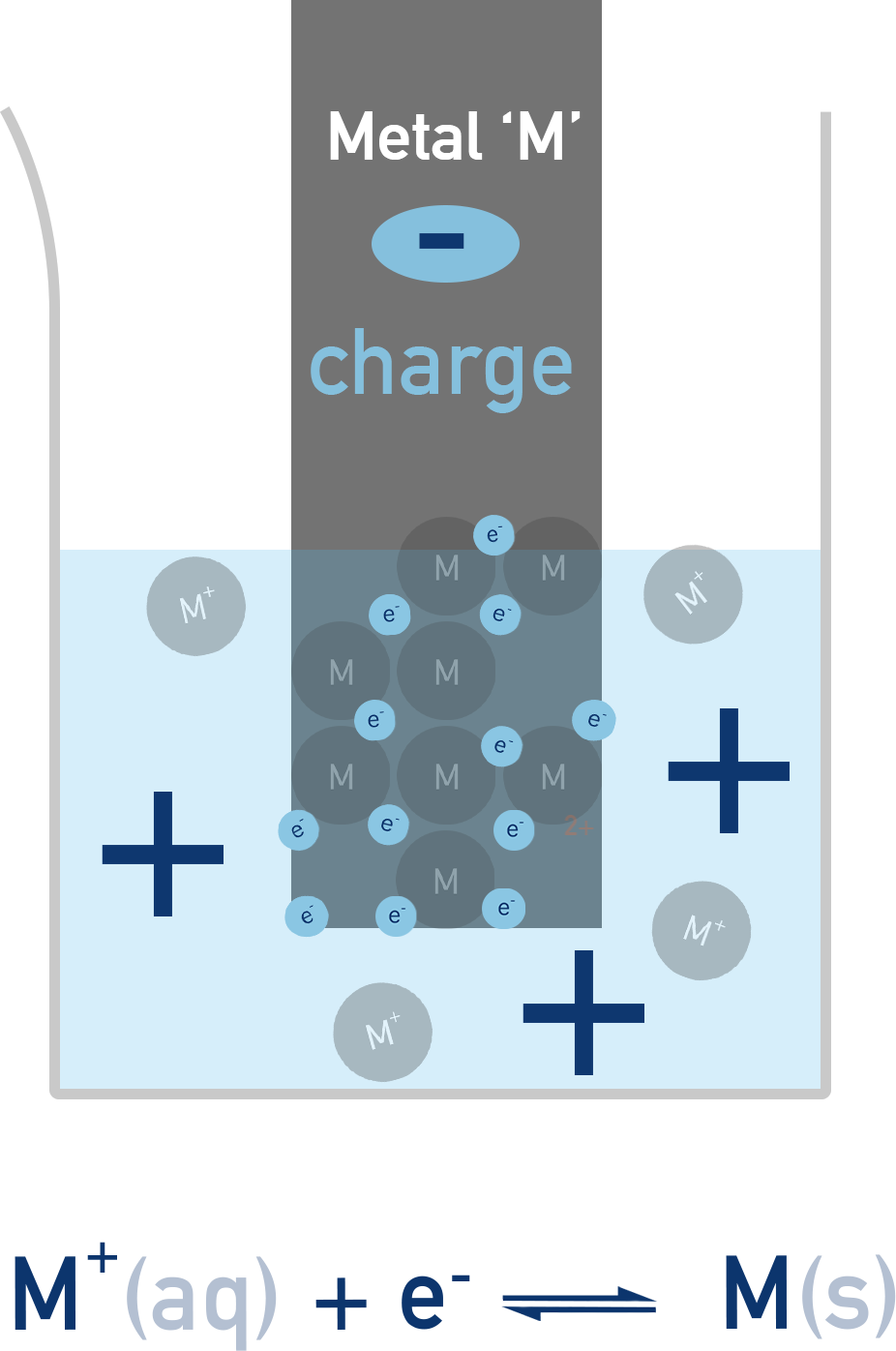
A redox equilibrium is established between the metal atoms and their ions:
Mⁿ⁺(aq) + ne⁻ ⇌ M(s)
This sets up a potential difference between the metal and the solution. The position of equilibrium – and hence the total charge or ‘potential’ of the electrode – depends on how readily the metal loses or gains electrons.
The electrode potential of a half-cell cannot be measured directly, but we can compare it to a standard reference (like the standard hydrogen electrode) to determine its relative value.
Components of a Voltaic Cell
The solid metal in each half-cell acts as an electrode, with one being the anode and the other the cathode.
- Anode: site of oxidation.
- Metal loses electrons and enters solution as ions.
- Electrons flow away from the anode.
- Cathode: site of reduction.
- Metal ions in solution gain electrons and are deposited as metal.
- Electrons flow into the cathode.
Salt Bridge Function
In a voltaic cell, the salt bridge allows ion exchange between half-cells. This prevents charge buildup by allowing:
- Cations to move toward the cathode
- Anions to move toward the anode
Salt bridge ions must be inert to avoid interfering with redox reactions, which is why potassium nitrate (KNO₃) is often used.
Because K+ and NO3- ions don’t easily get oxidised or reduced, meaning they don’t affect the redox processes occurring. They just simply allow charge to flow.
How a Simple Cell Works
Example: Zn–Cu Voltaic Cell
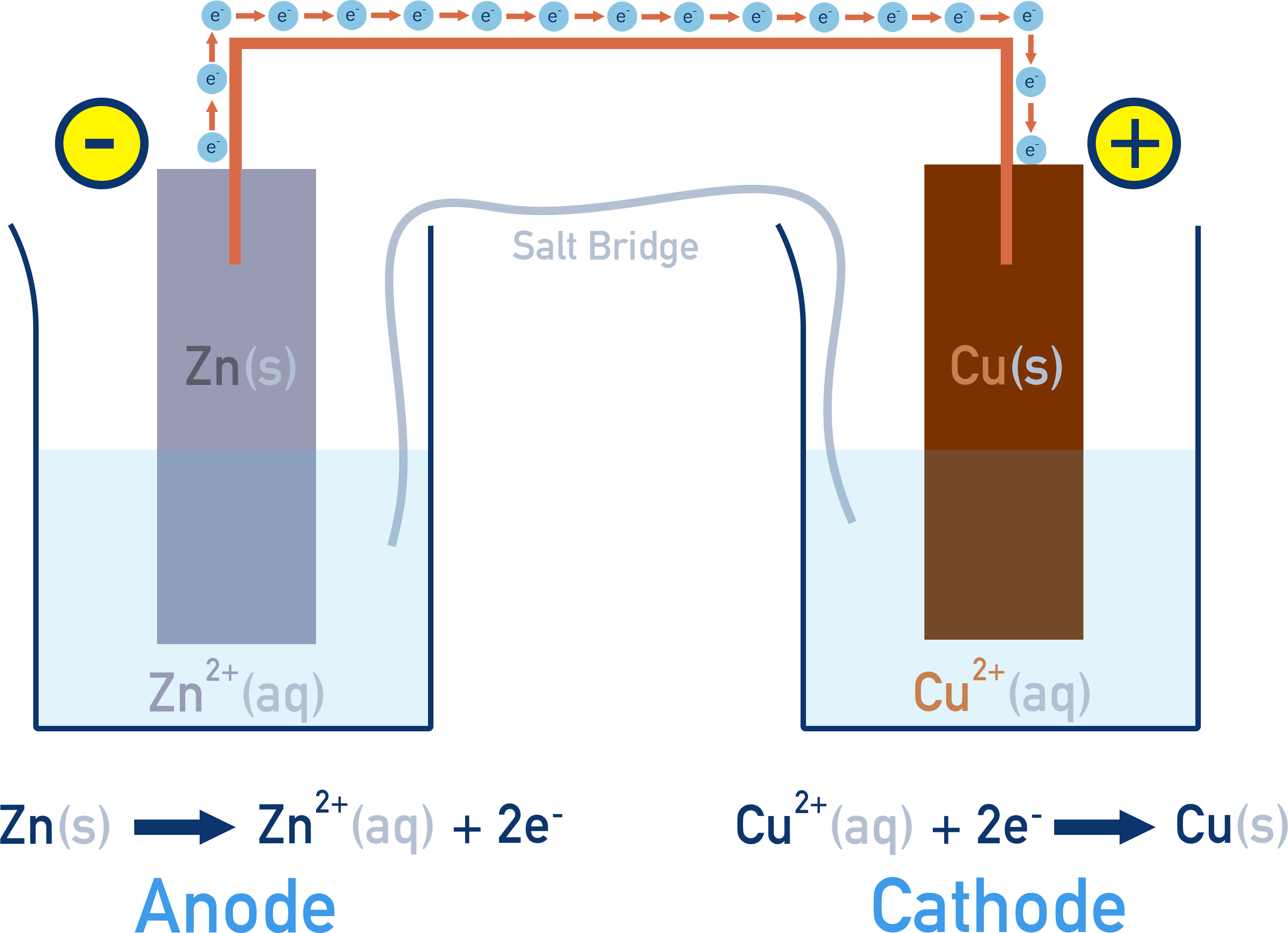
At the anode, zinc metal is oxidised: Zn(s) → Zn²⁺(aq) + 2e⁻
The released electrons travel through the external circuit to the cathode, where copper ions are reduced: Cu²⁺(aq) + 2e⁻ → Cu(s)
The salt bridge maintains charge balance by allowing ions to move between the two half-cells, preventing charge buildup.
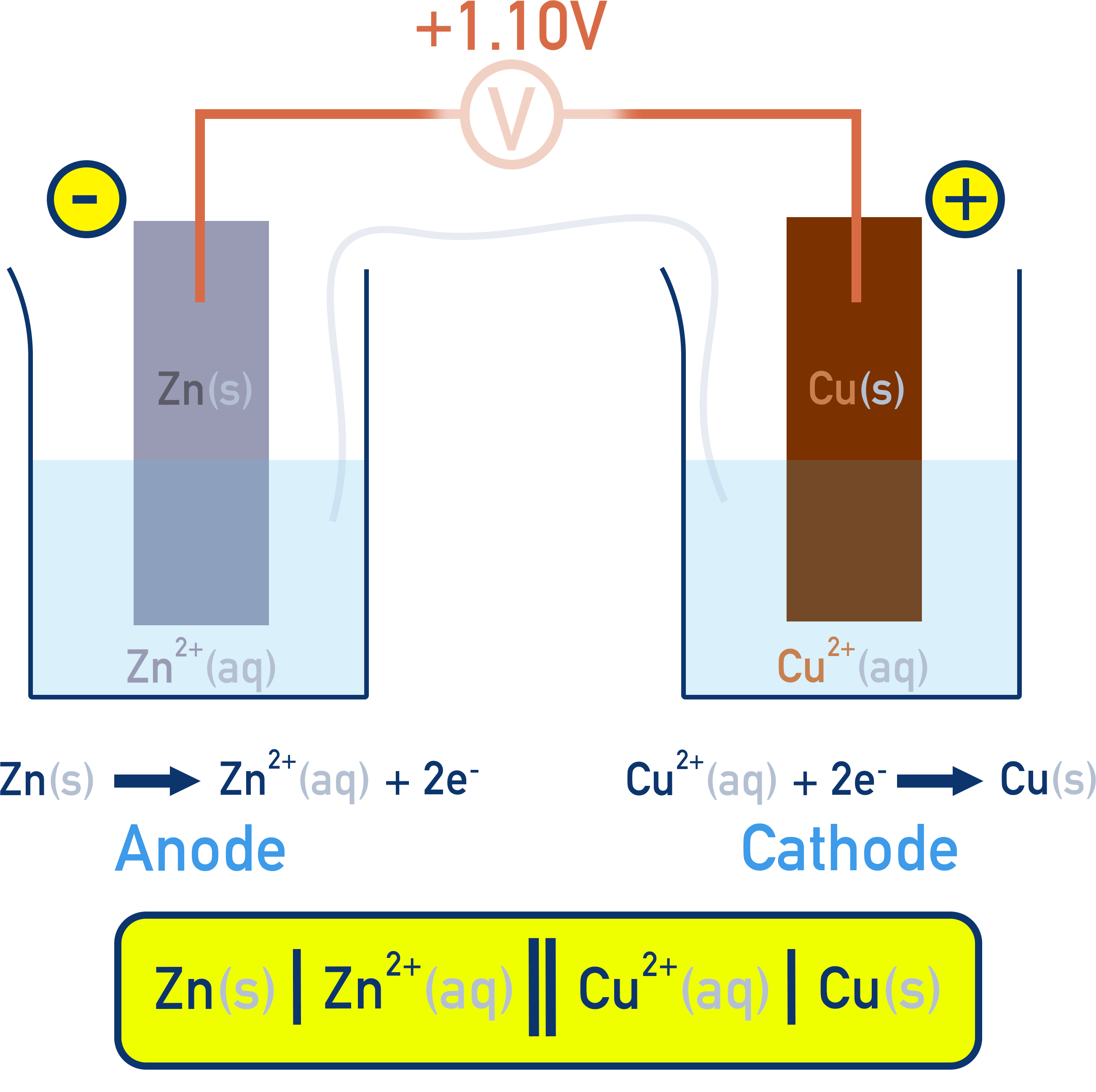
Cell Notation
Electrochemical cells can be written using shorthand notation:
- Single vertical line (|): separates different phases
- Double vertical line (||): salt bridge
- Anode (oxidation) on the left, cathode (reduction) on the right
Zn–Cu cell notation:
Zn(s) | Zn²⁺(aq) || Cu²⁺(aq) | Cu(s)
Non-Rechargeable Cells (Primary Cells)
In primary cells, the redox reactions are not reversible.
Once the active materials are used up, the cell can no longer generate current.
Example: Leclanché Cell (Zn/NH₄⁺)

- Anode: Zinc casing oxidised to Zn²⁺
- Cathode: Carbon rod where NH₄⁺ is reduced to NH₃
- Ammonia reacts with Zn²⁺ to form [Zn(NH₃)₄]²⁺, preventing gas buildup
Linked Course Question
What are the similarities and differences between energy from combustion and electrochemical reactions?
Similarities:
- Both involve redox reactions.
- Both can be used to generate electrical energy.
Differences:
| Combustion | Electrochemical Reactions |
|---|---|
| Redox happens in one step | Redox occurs in separate half-cells |
| Energy released as heat | Energy released as electrical current |
| Less efficient | More efficient (especially fuel cells) |
| Produces CO₂ and pollutants | Can be cleaner and more sustainable |
Summary
- Voltaic cells use spontaneous redox reactions to produce electricity.
- Electrons flow from anode (oxidation) to cathode (reduction).
- Salt bridge maintains electrical neutrality by ion movement.
- Zn–Cu cell is a classic example of a voltaic system.
- Primary cells are non-rechargeable and stop working once reactants are used.
