Shapes of molecules
Quick Notes
- Electron pairs around a central atom repel and arrange themselves as far apart as possible to minimise repulsion, giving different bonding shapes and arrangements.
- Repulsion order: Lone pair–lone pair > lone pair–bond pair > bond pair–bond pair.
- The shape of a molecule is determined by the number of bonding and lone pairs around the central atom.
- Common shapes and bond angles:
- Linear (180°) – e.g. CO2
- Bent (104°) – e.g. H2O
- Trigonal planar (120°) – e.g. BF3
- Trigonal pyramidal (107°) – e.g. NH3
- Tetrahedral (109.5°) – e.g. CH4
- Square planar (90°) – e.g. XeF4
- Trigonal bipyramidal (90°, 120°, 180°) – e.g. PCl5
- Octahedral (90°, 180°) – e.g. SF6
Full Notes
Electron Pair Repulsion Theory
The shape of a molecule can be predicted based on the number of bonds and lone pairs around the central atom. Bonds and lone pairs are considered regions of electron density.
- Bonding pairs and lone pairs repel each other due to their negative charge.
- To minimise repulsion, electron pairs arrange themselves as far apart as possible.
- Repulsion strengths follow this order:
- Lone pair–lone pair (LP–LP) repulsion → strongest repulsion
- Lone pair–bond pair (LP–BP) repulsion → intermediate repulsion
- Bond pair–bond pair (BP–BP) repulsion → weakest repulsion
- Lone pairs reduce bond angles by forcing bonding pairs closer together.
Common Molecular Shapes and Bond Angles
Quick Reference Summary Table at Bottom of Page
Linear (180°)
2 bonding pairs, no lone pairs → bonds remain in a straight line.

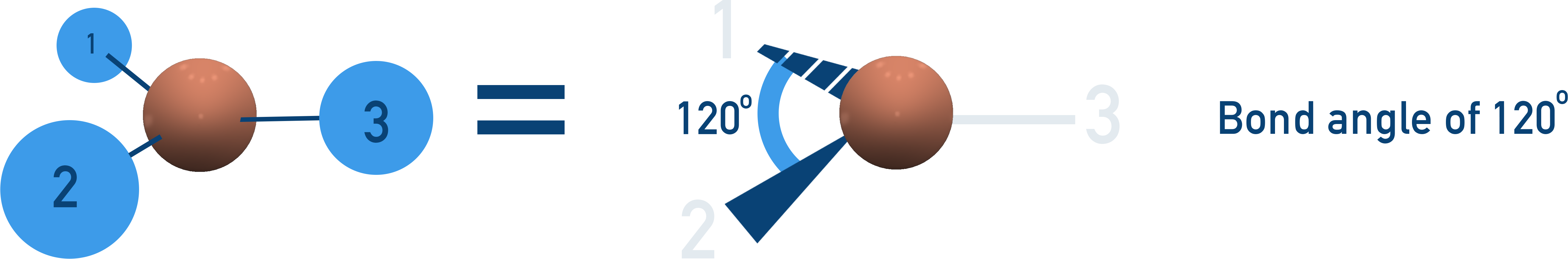
Trigonal Planar (120°)
3 bonding pairs, no lone pairs → flat triangle arrangement.
Examples: BF3, NO3−
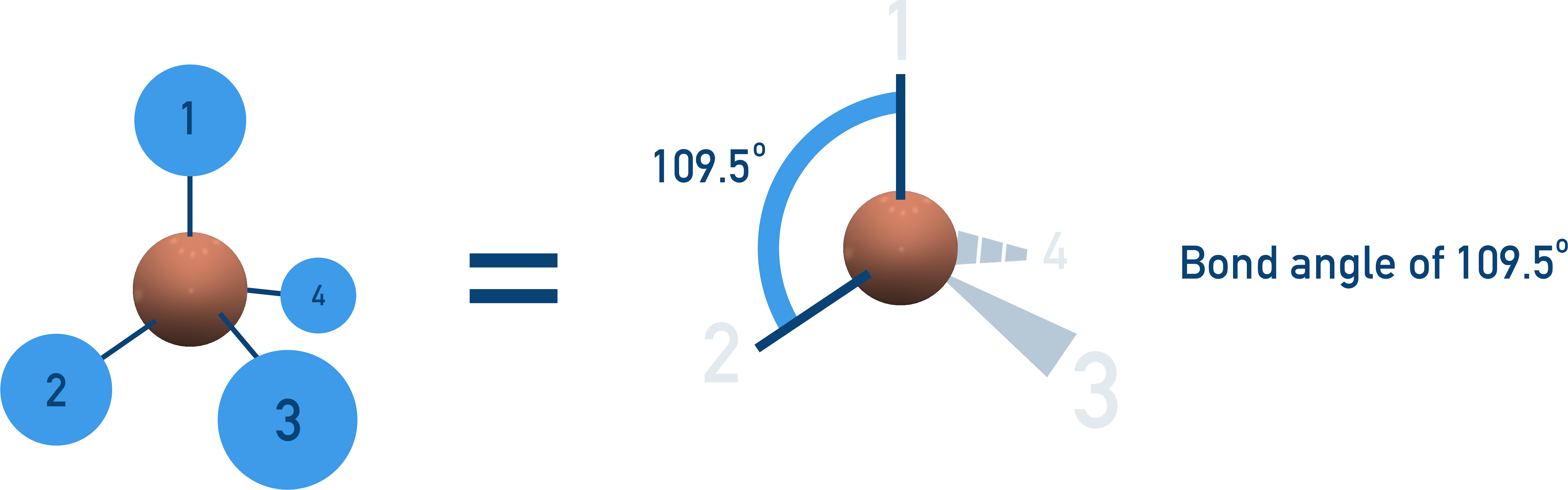
Tetrahedral (109.5°)
4 bonding pairs, no lone pairs → 3D tetrahedral shape.
Examples: CH4, NH4+
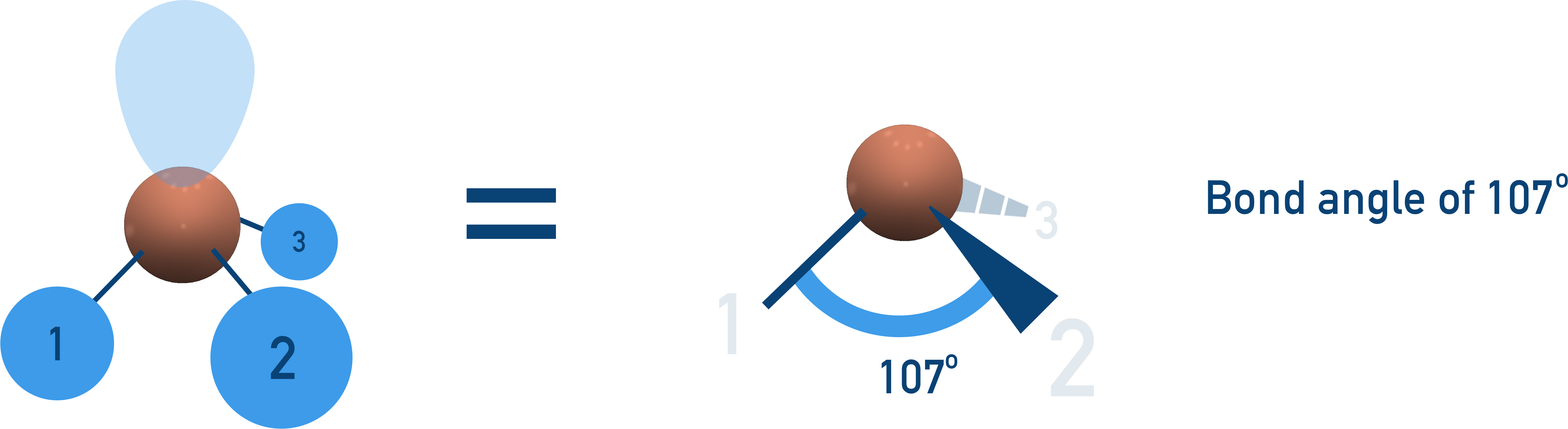
Trigonal Pyramidal (107°)
3 bonding pairs, 1 lone pair → bond angle reduced due to lone pair repulsion.
Examples: NH3, PCl3
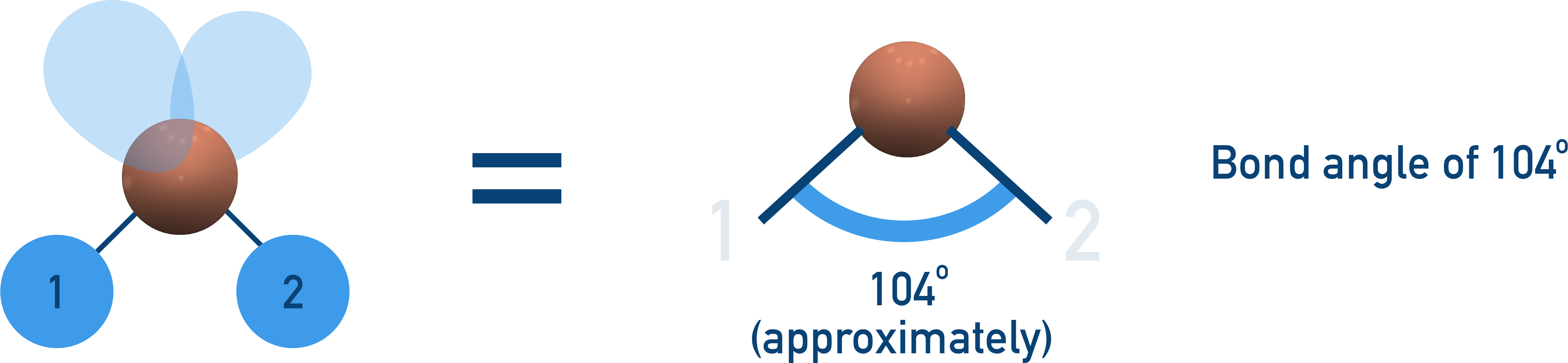
Bent (104.5°)
2 bonding pairs, 2 lone pairs → bond angle reduced further by two lone pairs.
Examples: H2O, OF2
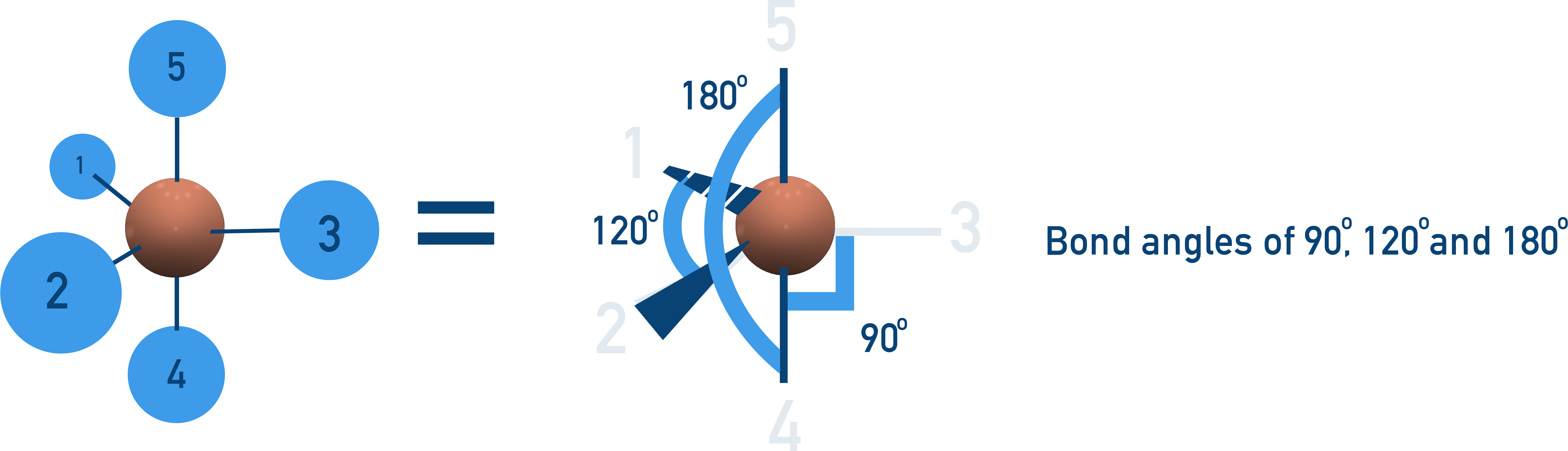
Trigonal Bipyramidal (90°, 120°, 180°)
5 bonding pairs, no lone pairs → atoms arranged in two layers.
Example: PCl5
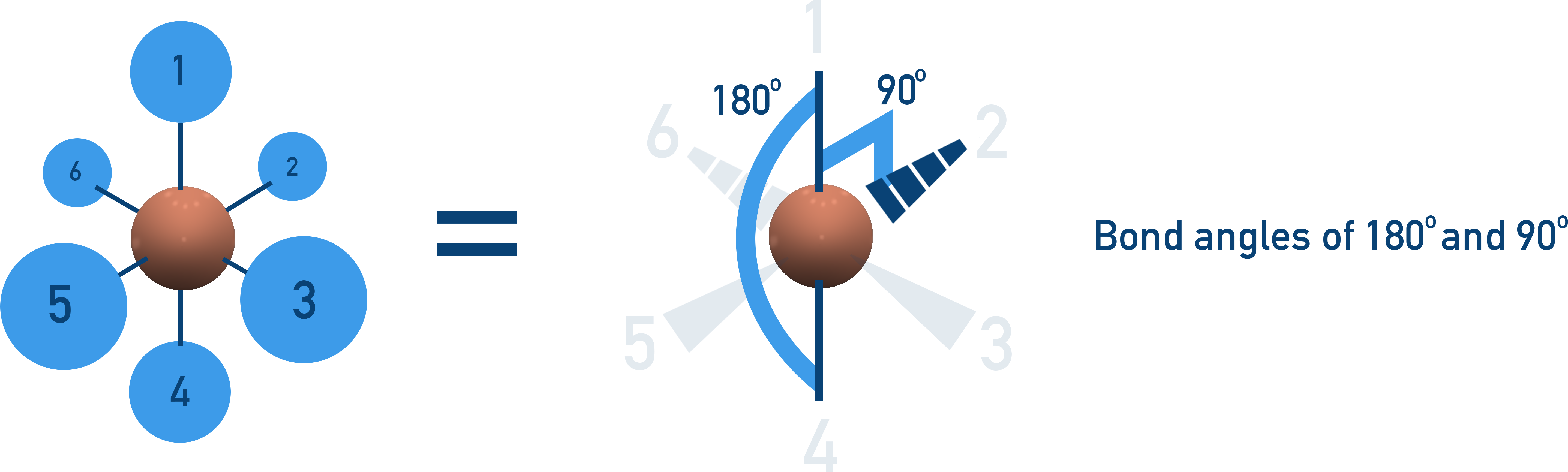
Octahedral (90°, 180°)
6 bonding pairs, no lone pairs → symmetrical 3D shape.
Example: SF6
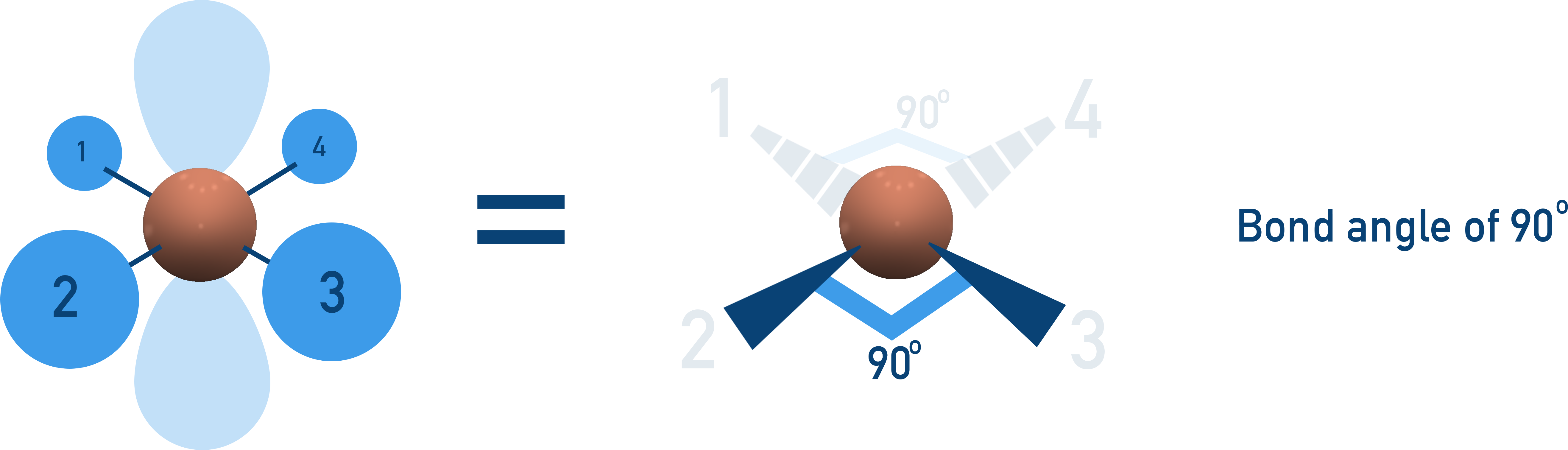
Square Planar (90°)
4 bonding pairs, 2 lone pairs → lone pairs opposite, minimising repulsion.
Example: XeF4
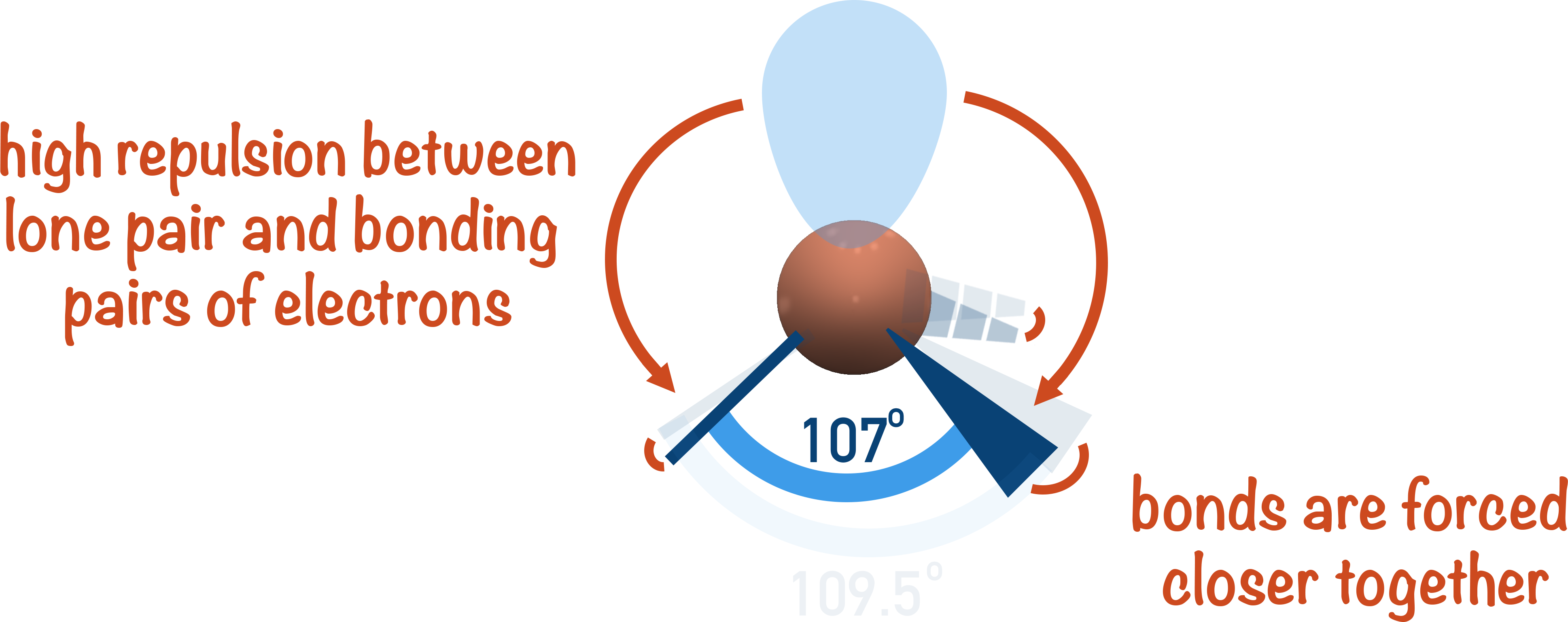
Effect of Lone Pairs on Bond Angles
Lone pairs repel bonding pairs more than bonding pairs repel each other. This pushes bonding pairs closer together and reduces bond angles.
| Lone Pairs Present | Bond Angle Reduction | Example |
|---|---|---|
| 0 | No reduction | CH4 (109.5°) |
| 1 | ~2.5° smaller | NH3 (107°) |
| 2 | ~5° smaller | H2O (104.5°) |
Molecular Shapes and Bond Angles – Key Examples
| Molecule | Electron Pair Geometry | Shape | Bond Angle(s) | Explanation |
|---|---|---|---|---|
| CO2 | 2 bonding pairs | Linear | 180° | No lone pairs, equal repulsion between bonds keeps atoms in a straight line |
| BF3 | 3 bonding pairs | Trigonal planar | 120° | Bonds spread evenly in one plane with equal repulsion |
| CH4 | 4 bonding pairs | Tetrahedral | 109.5° | Four bonds repel equally in 3D space, forming a symmetrical shape |
| NH3 | 3 bonding + 1 lone pair | Pyramidal | 107° | Lone pair pushes bonding pairs slightly closer together |
| H2O | 2 bonding + 2 lone pairs | Non-linear | 104.5° | Two lone pairs create even more repulsion, reducing angle further |
| PF5 | 5 bonding pairs | Trigonal bipyramidal | 120° (eq), 90° (ax) | Three bonds form a triangle in one plane; two others are perpendicular |
| SF6 | 6 bonding pairs | Octahedral | 90° | All 6 electron pairs repel equally, forming a symmetrical 3D shape |
Application in Ions
The same rules as above apply for polyatomic ions.
For Example:
- NH4+ → Tetrahedral (109.5°)
- NO3− → Trigonal Planar (120°)
- OH− → Bent (~104.5°)
Summary
| Shape | Bond Angle | Lone Pairs? | Example |
|---|---|---|---|
| Linear | 180° | No | CO2 |
| Trigonal Planar | 120° | No | BF3 |
| Tetrahedral | 109.5° | No | CH4 |
| Trigonal Pyramidal | 107° | 1 | NH3 |
| Bent (V‑Shaped) | 104.5° | 2 | H2O |
| Trigonal Bipyramidal | 90° & 120° | No | PCl5 |
| Seesaw | <90° & <120° | 1 | SF4 |
| T‑Shaped | <90° | 2 | ClF3 |
| Octahedral | 90° | No | SF6 |
| Square Pyramidal | <90° | 1 | BrF5 |
| Square Planar | 90° | 2 | XeF4 |
- Electron pairs repel and arrange as far apart as possible (VSEPR theory).
- Lone pairs repel more strongly than bonding pairs, reducing bond angles.
- Common shapes include linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, trigonal bipyramidal, octahedral, and square planar.
- These rules apply to both molecules and polyatomic ions.
